Uterine Cervix
 ANATOMY
ANATOMY
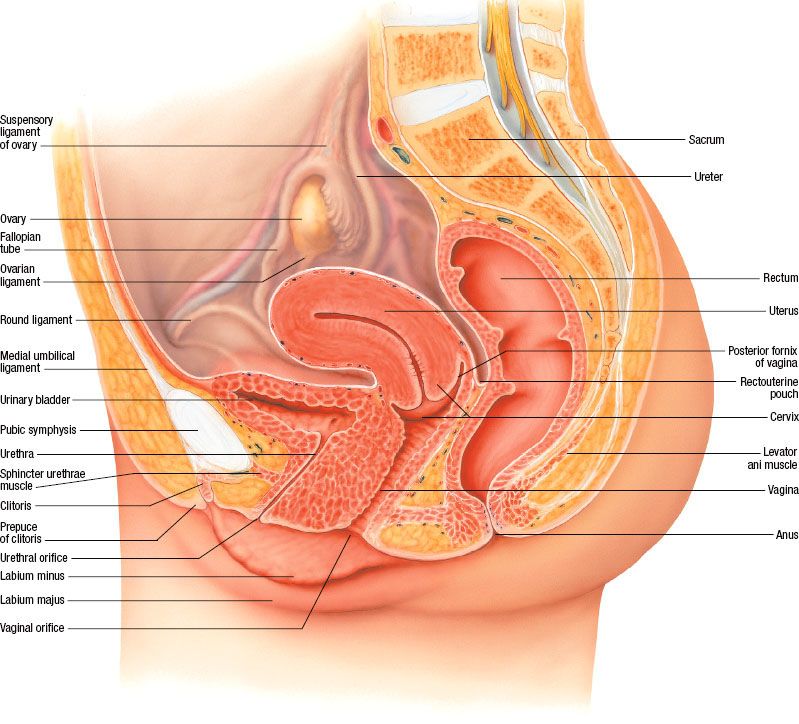
The uterus is a hollow, thick-walled, pear-shaped, muscular organ located in the pelvis above the vagina, behind the bladder, and in front of the rectum (Fig. 69.1). On average, it is approximately 7 to 8 cm long, 5 to 7 cm wide, and 2 to 3 cm thick. The uterus is divided into the uterine corpus superiorly and the uterine cervix inferiorly, with the most superior part of the corpus also known as the fundus and the middle portion of the corpus known as the body. The fundus is located superior to the line joining the entrance of the fallopian tubes. The body of the uterus is enclosed between layers of the broad ligament and is freely mobile. The regions of the body where the fallopian tubes enter are called the cornua. The most inferior, slightly constricted, portion of the uterus is called the isthmus or lower uterine segment (LUS). The cervix rests inferior to the LUS.
The uterus is usually bent anteriorly (anteflexed) between the cervix and the uterine body. The entire uterine-cervix structure is normally bent anteriorly (anteverted) in the pelvis. The uterus is frequently posteriorly retroverted, especially in older women who have a small uterus. The wall of the uterus has three layers: the outer serosal layer; the middle myometrium, which is approximately 12 to 15 mm of muscle through which the main blood vessels and nerves flow; and the inner coat called the endometrium.
The cervix measures approximately 3 by 3 cm and is predominantly a fibrous organ. The cervix is divided into an upper or supravaginal portion, above the ring containing the endocervical canal, and the vaginal portion, projecting in the vaginal vault. Central in the rounded vaginal region is the external os, bounded by the anterior and posterior lips of the cervix, extending inward to the internal os, the endocervical canal, and endometrial canal.
The uterus is partially covered by peritoneum in its fundal portion and posteriorly; its anterior and lateral surfaces are related to the bladder and the broad ligaments, respectively. It is attached to the surrounding structures in the pelvis by two pairs of ligaments—the broad and the round ligaments. The broad ligament is a double layer of peritoneum extending from the lateral margin of the uterus to the lateral wall of the pelvis. It contains the fallopian tubes. The two layers of peritoneum forming the broad ligament enclose the parametrium as it reaches the uterus. Inferiorly, the broad ligament follows the plane of the pelvic floor and ends medially in the upper portion of the vagina.1
The round ligament, a band of smooth muscle and connective tissue that contains small vessels and nerves, extends forward horizontally from its attachment in the anterolateral portion of the uterus to the lateral pelvic wall. The cord ascending from the lateral wall of the true pelvis crosses the pelvic brim and extends laterally to reach the abdominoinguinal ring, through which it leaves the abdomen to traverse the inguinal canal and terminates in the superficial fascia.
The uterosacral ligaments are paired supports for the lower uterus, extending from the uterus to the sacrum and running along the recto-uterine-peritoneal fields.1 The cardinal ligaments, also called transverse cervical ligaments (Mackenrodt’s), are thickened connective tissue and fascia arising at the upper lateral margins of the cervix and inserting into the fascial covering of the pelvic diaphragm.
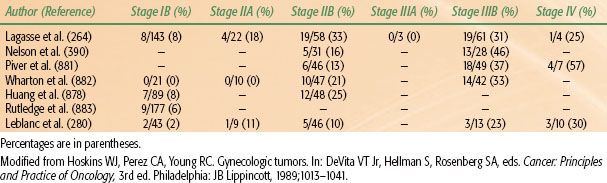
The uterus including the uterine cervix has a rich lymphatic network (Fig. 69.2) that drains principally into the paracervical lymph nodes; from there it goes to the external iliac (of which the obturator nodes are the innermost component) and the hypogastric lymph nodes. The pelvic lymphatics drain into the common iliac and the para-aortic lymph nodes. Lymphatics from the fundus pass laterally across the broad ligament continuous with those of the ovary, ascending along the ovarian vessels into the para-aortic lymph nodes. Some of the fundal lymphatics also drain into the common iliac lymph nodes. The main artery supplying the uterus is the uterine artery, which originates from the anterior division of the hypogastric artery.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Over the last 80 years, the morbidity and mortality of locally advanced invasive cervical cancer has dramatically declined in the United States and Europe due to effective screening and treatment of preinvasive lesions.2 In the United States 50% of women who develop cervical cancer have never been screened and another 10% have not been screened within the previous 5 years. However, in the last decade, incidence rates of invasive carcinoma have remained relatively constant. The American Cancer Society estimates that approximately 12,200 new cases of invasive carcinoma of the cervix arise in the United States and about 4,200 deaths will occur per year, in addition to >60,000 cases of carcinoma in situ.3
Worldwide, cervical cancer remains the most common gynecologic cancer and the third-most-common malignancy in women, with over 500,000 women globally developing this tumor and 233,000 dying of the disease every year.4 Unfortunately, it often affects young women, resulting in loss of the ability to bear future children. The larger societal impact from the death of young women in the prime of life and motherhood has not been measured.
In developing countries, cervical cancer is the leading cause of cancer-related death.2 Cervical cancer is more common in areas where women have less access to screening, including parts of Asia, Africa, and Central and South America. Whether regional differences in predisposition to developing cervical cancer exist is debated because it is impossible to adequately correct for unknown and known confounders, such as socioeconomic status, access to health care, parity, smoking, presence of other infections, immune status, and other factors affecting host immunity such as nutritional status.5
FIGURE 69.1. Anatomy of the pelvis. (Asset provided by the Anatomical Chart Company Lexington, SC.)
Human Papilloma Virus
Estimates indicate that >90% of cervical cancers are related to the presence of human papilloma virus (HPV) and are contracted via sexual intercourse.6 HPV is a small, double-stranded DNA virus; HPV 16 and 18, as well as a long list of other, less frequent subtypes, including but not limited to HPV 31, 33, 35, 39, 45, 51, 52, 56, and 58, have been well characterized as causative agents for cervical cancer, with some geographic variation.7 The HPV genome integrates into the host cell chromosomes in cervical epithelial cells and codes for six early and two late open reading frame proteins, of which three (E5, E6, and E7) alter cellular proliferation. Two viral genes, E6 and E7, are typically expressed in HPV-positive cervical-cancer cells. The E6 protein inactivates the major tumor suppressor p53; this causes chromosomal instability, inhibits apoptosis, and activates telomerase. The E7 protein affects the retinoblastoma protein (Rb), resulting in a loss of regulation of the cell’s proliferation and immortalization.8
Although a high prevalence of HPV exists worldwide, peaking at ages 25 to 35 years, <15% of exposed women develop persistent infection that results in dysplasia,9 whereas the majority of women clear the infection within 2 years.10 Cervical cancer may develop 10 to 20 years after initial exposure to HPV. Social factors related to cervical cancer include those associated with HPV transmission, such as early age of first intercourse; a history of multiple sexual partners; a male partner with a history of multiple sexual partners; a large number of pregnancies;11,12 and a history of sexually transmitted disease, including gonorrhea, chlamydia,13 herpes simplex virus II, and/or human immunodeficiency virus (HIV).14 A higher incidence of cervical cancer exists among women whose spouses are known or suspected to have had higher exposure to HPV15 or whose partners have a history of penile carcinoma.16,17 Whether circumcision may be protective to women is controversial18 because circumcision may be a surrogate for unknown factors related to HPV transmission.19
Chemical,20 hormonal, or other carcinogens21 may be implicated in cervical cancer. An association between cervical carcinoma and oral contraceptive use has been reported but is considered controversial.22 Prenatal exposure to diethylstilbestrol (DES) is linked to the development of clear-cell adenocarcinoma, although the overall incidence is small (0.14 to 1.4 per 1,000 DES-exposed women).23–25 Cigarette smoking may increase the risk of cervical cancer.26 However, passive smoking may not be an independent factor in the absence of active smoking.27 A review of >50 studies considers smoking a cofactor for HPV infection and carcinogenesis,28 although one study does not confirm this.15 Current smoking (relative risk [RR] = 1.55) and younger age at HPV exposure (RR = 1.75) are considered risk factors among HIV-positive women.29 Intrauterine device use may decrease cervical cancer risk, potentially through an increase in cellular immunity triggered by the device.30
HPV Vaccination
The quadrivalent human papillomavirus recombinant vaccine for HPV types 6, 11, 16, and 18, first approved in the United States in 2006 for girls and women ages 9 to 26 years, is now available for boys ages 9 to 26 years, with the goal of eradicating HPV-related gynecologic, penile, anal, and oropharyngeal cancers. A second vaccine with strong immunogenicity to HPV types 16 and 18, approved for girls 9 to 25 years old, is more frequently administered in Europe. Although its development is a major advance in the prevention of cancer, vaccine implementation has been hindered worldwide by cost and access. With an increase in understanding and availability, the hope is that all children will be given a vaccine covering all subtypes in the future.
FIGURE 69.2. A: Lymph vessels and lymph nodes of the cervix and the body of the uterus. (Asset provided by the Anatomical Chart Company, Lexington, SC.) B: Three-dimensional reconstruction of location of pelvic and common iliac lymph nodes outlined on computed tomography scans in patients with carcinoma involving the distant vagina. Treatment portal is shown. C: The incidence of cervical cancer increased slightly in the U.S. from 2005–2010.
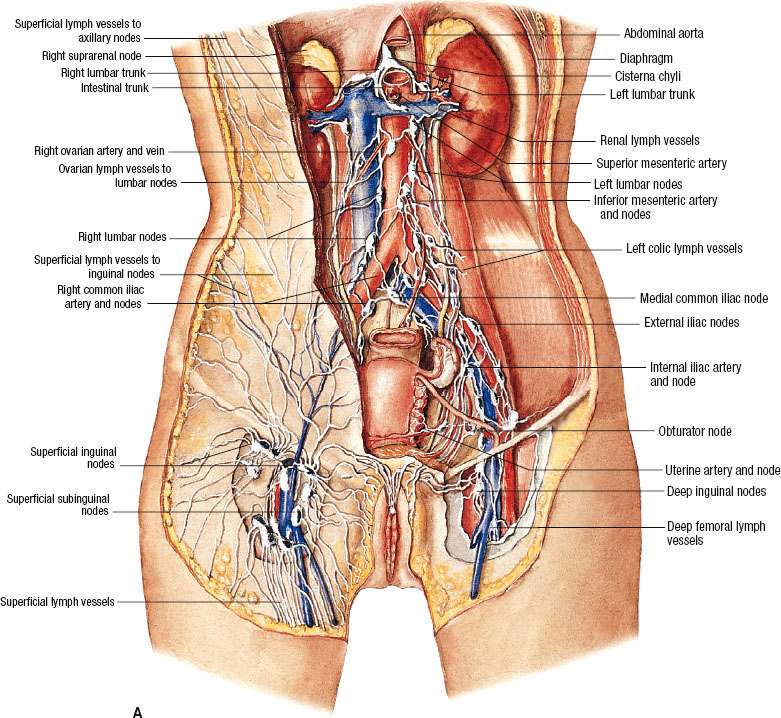
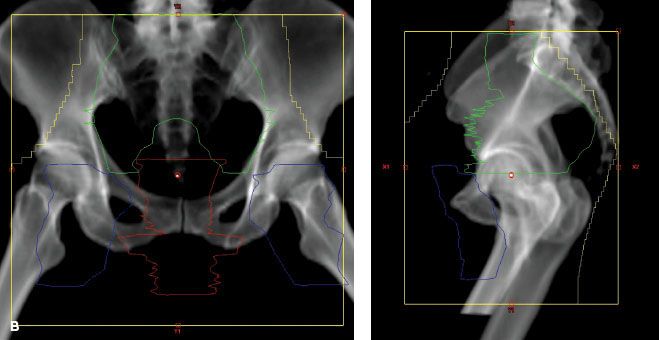
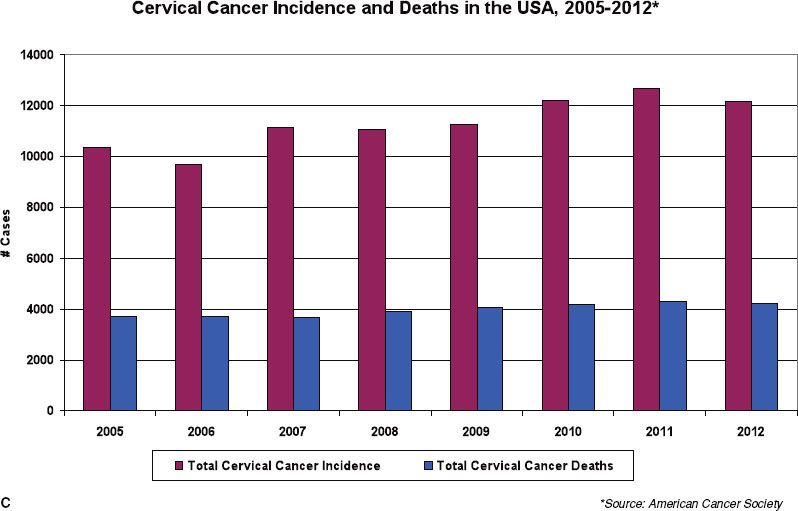
TABLE 69.1 INCIDENCE OF PELVIC NODE METASTASES IN CARCINOMA OF THE UTERINE CERVIX
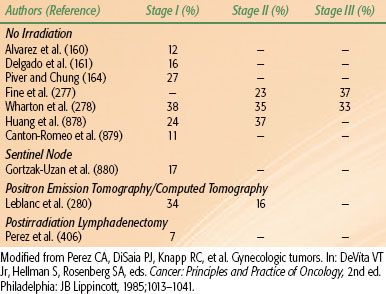
TABLE 69.2 METASTASES TO PARA-AORTIC LYMPH NODES IN CARCINOMA OF THE UTERINE CERVIX
 NATURAL HISTORY AND PATTERNS OF SPREAD
NATURAL HISTORY AND PATTERNS OF SPREAD
Squamous cell carcinoma of the uterine cervix usually originates at the squamous columnar junction (transformation zone) of the endocervical canal and the portio of the cervix.
Cellular transformation follows a stepwise progression from normal to higher levels of dysplasia. Of patients diagnosed with cervical intraepithelial neoplasia (CIN) type 1, 60% have regression of the lesion, and of those with CIN2, 40% regress. Higher levels of dysplasia are more likely to progress to cancer, particularly in the presence of cofactors such as smoking or impaired immunity. Although progression typically takes 10 to 20 years,31,32 in some instances a rapid development of carcinoma may be associated with aggressive disease.
The development of a malignant phenotype results from cells that break through the basement membrane of the epithelium and invade the cervical stroma. Invasion may result in spread to pelvic lymph nodes or other, more distant sites.33 If the cells are detected at this stage by a Papanicolaou (Pap) or thin preparation test, appropriate minimally invasive therapy may suffice. However, if the lesion progresses, it may present as a superficial ulceration or exophytic tumor in the ectocervix or with extensive infiltration of the endocervix. If untreated, the tumor may spread to the adjacent vaginal fornices, paracervical or parametrial tissues,34 or adjacent organs, including the bladder, the rectum, or both. Landoni et al.35 studied 230 patients with clinical stages IB and IIA tumors treated with radical hysterectomy with pelvic lymphadenectomy and noted that the tumor spread endocervically equally in all directions. Tumor extension into the vesicocervical ligament (anterior parametrium) was noted in 23% of cases, into the uterosacral ligaments (posterior parametrium) and the rectovaginal septum in approximately 15%, and into the parametria in 28% to 34% of cases. Paracervical extension was related to the depth of stromal invasion, tumor size, lymphatic invasion, and presence of lymph node metastasis.
Approximately 10% to 30% of patients with carcinoma of the uterine cervix have extension into the lower uterine segment and the endometrial cavity.36 Decreased survival rates and a greater incidence of distant metastases were reported by Perez et al.36 and Chao et al.37 in patients with stromal endometrial invasion or replacement of normal endometrium by cervical carcinoma. Regional lymphatic or hematogenous spread may occur and increases with stage, although dissemination does not always follow an orderly sequence, and occasionally a small primary tumor may be seen infiltrating the pelvic lymph nodes, invading the bladder or rectum, or metastasizing distantly.
Both adjacent parametrial and pelvic lymph nodes may be involved. Girardi et al.38 analyzed 359 radical hysterectomy specimens and found positive parametrial nodes in 280 patients (78%); the incidence of positive nodes was 11.4% in stage IB and 21.5% in stage IIB disease. With negative parametrial nodes, only 26% of patients had positive iliac lymph nodes, whereas 81% of patients with positive parametrial lymph nodes also had pelvic-node metastases. These data underscore the need to irradiate the parametrial tissues or carry out a complete bilateral pelvic lymphadenectomy with a radical hysterectomy in patients with invasive cervical carcinoma.
Spread of carcinoma of the cervix may progress to the obturator lymph nodes, considered a medial group of the external iliac chain, to other external iliac nodes, and to the hypogastric lymph nodes. From these, there may be tumor metastases to the common iliac or para-aortic lymph nodes.39 The incidence of metastasis to pelvic or para-aortic lymph nodes for various stages of the disease is listed in Tables 69.1 and 69.2. In one study, pelvic lymph nodes were dissected in 225 patients with cervical carcinoma treated with radical hysterectomy; positive pelvic nodes were identified in 13 of 91 women (14.2%) with stages IB and IIA, 16 of 81 (19.8%) with stage IIB, and 11 of 40 (28%) with stage IIIB disease.40 The most commonly involved groups were the parametrial, obturator, external iliac, and common iliac nodes (Fig. 69.3). Para-aortic lymph nodes were involved in 3 of 91 patients (3.3%) with stage IB or IIA tumors 4 cm or less and in 5 of 38 patients (13.1%) with stage IIB or III disease.
Spread through the venous plexus and the paracervical veins resulting in hematogenous dissemination, though infrequent, is relatively common with more advanced stages. In an analysis of 322 patients in whom distant metastases developed, the most frequently observed metastatic sites were the lung (21%), para-aortic lymph nodes (11%), abdominal cavity (8%), and supraclavicular lymph nodes (7%).41 Bone metastases occurred in 16% of patients, most commonly to the lumbar and thoracic spine (Table 69.3). Spinal epidural compression from metastatic tumor, often involving lumbar segments, can occur rarely,42 and metastasis to the brain and the heart have been reported, although it is unusual to have spread to the brain without evidence of pulmonary metastases already present,43,44 even for small-cell carcinoma of the cervix.45
FIGURE 69.3. Distribution of pelvic node metastases in 14 patients with stages IB to IIA cervical cancer, tumor size <4 cm (A), and 38 patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy (B). (From Benedetti-Panici P, Maneschi F, Scambia G, et al. Lymphatic spread of cervical cancer: an anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol Oncol 1996;62:19–24; with permission from Elsevier.)
 PAP SMEAR SCREENING
PAP SMEAR SCREENING
The American College of Obstetrics and Gynecology guidelines published in 200946 state that Pap smear screening should begin at age 21 years and continue every 2 years until age 30 years; then, if there are three normal consecutive Pap smears and no history of CIN2, CIN3, DES exposure, or HIV infection and the woman is not otherwise immunocompromised, screening should be every 3 years. Women who have had a hysterectomy for benign reasons and have no history of high-grade squamous intraepithelial lesion may discontinue testing. Co-testing of the Pap smear with an HPV DNA test is appropriate for low-risk women older than age 30 years. If negative, rescreening is not required sooner than 3 years. Women who have been treated for CIN2 or CIN3 need annual screening for at least 20 years. Those who have had a hysterectomy and a history of CIN2/CIN3 should continue to undergo screening with annual pelvic exams.
When obtaining the Pap smear, special attention should be directed to not using a lubricating agent (warm water on the speculum will suffice), to obtaining good “scrapings” from the cervix and vaginal posterior fornix (without blood), and to using a small brush to obtain an endocervical sample. The patient should be instructed not to cleanse with a douche before the examination, and, if indicated, specimens should be obtained to check for trichomonas. If the cytologic smear shows atypia or mild dysplasia (class II), it should be repeated no sooner than 2 weeks after the initial test to allow representative cellular exfoliation to occur. Guidelines for reporting results of cervical and vaginal cytology were promulgated in 1988. The Bethesda system eliminated the classes of Pap cytology. The correlation between the cytologic diagnosis and subsequent histologic examination is >90%.47 This system was modified in 1991 and in 2001.48
TABLE 69.3 CARCINOMA OF THE UTERINE CERVIX (MALLINCKRODT INSTITUTE OF RADIOLOGY 1959–1986): ANATOMIC SITE OF FIRST METASTASIS
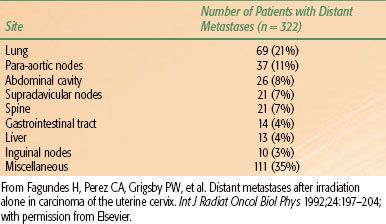
TABLE 69.4 DIAGNOSTIC WORKUP FOR CARCINOMA OF THE UTERINE CERVIX

 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Cervical cancer in the United States is most frequently identified during routine gynecologic examination. Intraepithelial or early invasive carcinoma of the cervix may be detected by cytologic smears before symptoms appear; Pap smear, colposcopy and biopsies, and HPV testing have high specificity and sensitivity. Visible lesions present with an exophytic mass or a barrel-shaped cervix due to an endocervical lesion. Patients may present complaining of metrorrhagia (intermenstrual bleeding), menorrhagia (heavier menstrual flow), or postcoital bleeding. If chronic bleeding occurs, the patient may complain of fatigue or other symptoms related to anemia.
In cases with more advanced disease, bowel obstruction, renal failure, foul-smelling serosanguinous or yellowish vaginal discharge, pelvic pain, flank and/or leg pain, rectal bleeding, obstipation, dysuria, hematuria, or persistent edema of lower extremities due to lymphatic/venous blockade by pelvic sidewall disease may occur. Pain in the pelvis or hypogastrium may be caused by tumor necrosis or associated pelvic inflammatory disease. In patients with pain in the lumbosacral area, the possibility of para-aortic lymph node involvement with extension into the lumbosacral roots or hydronephrosis should be considered.
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
When a patient presents with an abnormal smear or if abnormal squamous cells of undetermined significance (ASCUS) are detected but HPV status is negative, follow-up in 1 year is recommended. If the second smear reveals ASCUS, regardless of HPV status, colposcopy is recommended. When both ASCUS and HPV are present or adenocarcinoma in situ or a squamous intraepithelial lesion is identified, directed biopsies at the time of colposcopy should be carried out. Endocervical curettage may be performed except in pregnant women. If the biopsy results are negative, the procedure should be repeated in 6 months, and, if they are positive, a conization should be performed.
Patients who present with a clinically visible lesion should be jointly evaluated by the radiation and gynecologic oncologists. After obtaining a careful clinical history and performing a general physical examination, with attention to the inguinal and supraclavicular (nodal) areas, abdomen, and liver, a careful pelvic examination should be carried out with as little discomfort to the patient as possible without compromising the thoroughness of the evaluation.49 Pelvic examination should include inspection of the external genitalia, vagina, and uterine cervix, a rectal examination, and bimanual palpation of the pelvis. Pelvic examination under anesthesia is a universally accepted component in the evaluation and clinical staging of patients in order to provide a pain-free examination that allows a clearer estimation of parametrial or sidewall tumor extension. In countries in which magnetic resonance imaging (MRI) is available, this may be used to assist with assessing tumor extension beyond the lower cervix, and in many institutions it has replaced the examination under anesthesia. Cystoscopy or rectosigmoidoscopy should be performed in all patients with symptoms consistent with presence of a fistula of the urinary or lower gastrointestinal tract, patients with clinical stage IIB, III, or IVA disease who cannot undergo an MRI, or patients with an MRI suspicious for bladder or bowel invasion. The diagnostic procedures for carcinoma of the cervix are presented in Table 69.4.
Conization/Loop Excision
Conization involves a conical removal of a large portion of the ectocervix and endocervix. Cold knife cone biopsy specimens should always be obtained with a scalpel or other appropriate instrument. At least 50% of the endocervical canal should be removed without compromising the internal sphincter. Curettage of the remaining endocervical canal should be carried out.
Conization must be performed in the following situations: no gross lesion of the cervix is noted and an endocervical tumor is suspected; the entire lesion cannot be seen with the colposcope; diagnosis of microinvasive carcinoma is made on biopsy; discrepancies are found between the cytologic and the histologic appearances of the lesion; or the patient is not reliable for all necessary follow-up. With careful selection of patients who have a negative positron emission tomography (PET) scan and an MRI with a central lesion <2 cm in width, knife conization with lymphadenectomy may be considered for fertility preservation. Laser conization and loop diathermy excision are frequently done in an office setting as an alternative to conization; loop excision is less expensive and more reliable than laser conization.
Biopsy
Multiple punch biopsies of a grossly visible lesion should be adequate to confirm the diagnosis of invasive carcinoma. Specimens should be obtained from any suspect area and from all four quadrants of the cervix and from any suspect areas in the vagina. It is important to obtain tissue from the periphery of the lesion with some adjoining normal tissue; biopsy specimens from central ulcerated or necrotic areas may not be adequate for diagnosis. Dilation and curettage is not required if the biopsy confirms a diagnosis of invasive disease.
TABLE 69.5 COMPUTED TOMOGRAPHY AND POSITRON EMISSION TOMOGRAPHY IN THE EVALUATION OF PARA-AORTIC NODES
Laboratory Studies
For invasive carcinoma, patients should have the following laboratory studies: complete peripheral blood evaluation, including hemogram, white blood cell count, differential and platelet count; blood chemistry profile, with particular attention to blood urea nitrogen and creatinine; liver function values; and urinalysis.
Imaging Studies
In countries where three-dimensional (3D) imaging is not routinely available, patients with cervical cancer should have a chest radiograph to assess for lung metastases and an intravenous pyelogram (IVP) to determine whether hydronephrosis is present. The IVP in many countries has been replaced by computed tomography (CT) scan of the pelvis and abdomen with intravenous (IV) contrast material or by PET/CT scan. In places where CT is not available, patients with stages IIB, III, and IVA disease who have symptoms in the colon and rectum may benefit from a barium enema. A skeletal survey may be performed to determine whether bone metastases are present. Historically, pedal lymphangiography was used to assess lymph node involvement in the pelvic or para-aortic nodes with mixed results.50,51 The number of physicians trained to perform lymphangiography has declined in the United States, and instead PET scan is preferred.
Since the 1990s, the use of CT scanning has rapidly increased worldwide. A CT provides diagnostic information about the presence of metastases, enlarged lymph nodes, and the primary tumor. On a CT scan, the cervical tumor may be seen as an enlarged, irregular, hypoechoic cervix or as a mass with ill-defined margins. Parametrial regions appear dense when involved, and uterosacral involvement may be seen. Lymph nodes appear enlarged, with most >1 cm on axial dimension considered pathologic. The overall accuracy of CT scanning in staging cervical cancer ranges from 63% to 88%.50,52 In the detection of lymph node abnormalities, the overall accuracy of conventional CT scanning is 77% to 85%, with sensitivity of 44% and specificity of 93%.53
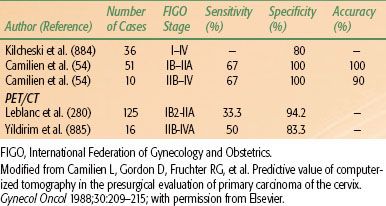
In order to correlate radiographic and surgical findings, Camilien et al.54 reported on 61 patients with carcinoma of the cervix who had both preoperative CT scans and exploratory laparotomy; results showed that 75% of the enlarged pelvic lymph nodes on CT contained metastases, and 97% of patients with negative nodes on CT scan had pathologically negative findings (specificity of 97%). However, histologically positive pelvic nodes were often missed on CT scan (sensitivity of 25%). The CT scan is more valuable in evaluation of the para-aortic lymph nodes (specificity of 100% and sensitivity of 67%).
Several studies evaluated the role of CT or PET/CT with regard to para-aortic nodal detection (Table 69.5). Heller et al.55 conducted a prospective evaluation of 320 patients with stages IIB to IVA carcinoma of the cervix entered into a Gynecologic Oncology Group (GOG) protocol in which preoperative CT scan, lymphangiography, and ultrasonography of the aortic area were performed. Para-aortic node dissection was done in patients with negative staging studies. Lymphangiography, CT scan, and ultrasonography had false-negative frequencies for pelvic lymph node evaluation of 14.2%, 25%, and 30%, respectively. The sensitivity was 79% for lymphangiography, 34% for CT scan, and 19% for ultrasonography, and the specificity ratings were 73%, 96%, and 99%, respectively. Ultrasonography, therefore, is not reliable in preoperative detection of lymph node metastases, but it has limited value in evaluating extrauterine tumor involvement. Ultrasound has a primary role in assisting with intracavitary brachytherapy applicator insertion and may detect uterine perforation, allowing for proper positioning, which is critical for adequate dosing and affects survival.56,57 PET has a higher sensitivity than CT and higher specificity than MR in detecting bone metastases.58
Magnetic Resonance Imaging
MRI is frequently used for the initial assessment of the cervical tumor and of extracervical tumor extension,59 often in lieu of an examination under anesthesia. MRI is contraindicated in patients with pacemakers, cochlear implants, metallic prostheses, metallic fragments from prior accidents, or large vascular clips. On T2-weighted images, a cervical cancer may be seen as a mass of intermediate to high signal intensity, usually of greater intensity than the fibrocervical stroma. On T1-weighted images, tumors are usually isointense with the normal cervix and may not be seen60 but can increase in intensity with the administration of IV contrast. Abnormal, irregular cervical margins, prominent parametrial strands, exocentric parametrial enlargement, and loss of parametrial fat planes on T1-weighted images or high signal in the parametria or cardinal/uterosacral ligaments on T2-weighted images are indicative of more extensive tumors.52,59,61 Parametrial tumor may be identified as brighter regions on T2-weighted images when compared to the low signal intensity of the cervix and uterine ligaments.
A comparative evaluation of pretreatment tumor staging and volume as assessed by examination under anesthesia (EUA), transrectal ultrasonography (TRUS), and MRI in 60 patients with invasive carcinoma of the cervix was reported by Hawnaur et al.62 TRUS and MRI assigned the same tumor stage in only 30% of patients, and EUA and MRI agreed on tumor stage in an additional 27%. In cases of disagreement, the MRI staging correlated better with outcome than TRUS or EUA. Sixty-two percent of patients with enlarged lymph nodes on pretreatment MRI either died or had tumor recurrence or metastases. MRI was superior to both TRUS and EUA in assessing the full extent of bulky tumors and lymph node enlargement.
Postema et al.63 compared MRI with pelvic examination (including under general anesthesia in selected patients) and surgicopathologic findings in 103 patients with invasive cervical carcinoma. MRI was better at identifying extracervical tumor spread, but it had more false-positive results. The pelvic examination led to correct treatment decisions in 89% of patients. In a study by Hansen et al.,64 clinical assessment (done according to International Federation of Gynecology and Obstetrics [FIGO] recommendations) was superior to low-field MRI with contrast enhancement in staging cervical cancer in 95 women who had both within 2 weeks after clinical diagnosis; the clinical staging correctly classified 57 patients (accuracy, 92%) compared with 52 for MRI (accuracy, 84%).
A prospective study by the American College of Radiology Imaging Network compared clinical examination, CT, and MRI.53 MRI was significantly better than clinical examination or CT for detecting uterine-body involvement or measuring tumor size,65 but no method was accurate at evaluating the cervical stroma. MRI was significantly better66 at detecting the tumor and parametrial involvement. MRI also somewhat increased detection of involved lymph nodes.53
The tumor is less likely to be as visible on MRI for adenocarcinoma cases, compared to squamous cell cancer. Haider et al.67 evaluated 56 patients with adenocarcinoma involving the cervix using MRI and noted that 42 (75%) had a visible mass. Kodaira et al.68 reviewed records of 84 patients with stage II cancer evaluated by MRI. The 5-year disease-free survival (DFS) rate of patients with maximal tumor size (Dmax) of ≥50 mm was significantly lower than that for patients with Dmax < 50 mm (46% vs. 88%; p < .0001).
Ebner et al.69 reviewed MRI findings in 12 women with recurrent pelvic tumors and 10 with a fibrotic mass (confirmed by laparotomy or biopsy in 21 patients). They were able to differentiate between the two processes accurately in most instances. However, it is highly desirable to confirm abnormal or suspect lymph node radiographic findings with CT-guided fine-needle aspiration biopsies.
Corn et al.70 evaluated endorectal coil MRI in 18 patients with stages IB to IIIB cervical carcinoma; in 7 patients, tumors were a higher stage by endorectal coil MRI because of proximal vaginal involvement or the combination of proximal vaginal involvement and parametrial extension. Compared with those who had a dark or intermediate signal, patients with bright signal characteristics tended to present with earlier stages, were less likely to have anemia, and were more likely to have complete response to external-beam radiation.
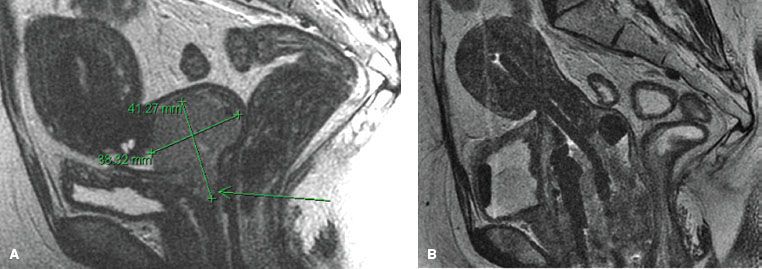
Radiation-induced changes detected over the course of radiation may predict local recurrence and survival. Mayr et al.71 studied 34 patients with cervical cancer of various FIGO stages who underwent 1.5-T MRI before and after radiation therapy. Tumor volumetry (3D measurements) based on T2-weighted images quantified the tumor regression rate. Sequential tumor volumetry using MR imaging may be a very effective measure of the responsiveness of cervical cancer to irradiation. MRI dynamic contrast enhancement during the first 2 weeks of radiation therapy may provide early prediction of tumor regression rate. In 7 patients, tumor regression rates ranged from 2% to 15.2% per day and correlated positively with changes in both peak and mean tumor enhancement (p < .01). Hatano et al.72 evaluated MRI in 42 patients with advanced cervical cancer treated with external-beam irradiation and high–dose-rate (HDR) brachytherapy. In biopsies performed immediately after radiation therapy (RT), no residual cancer was found in 36 patients (86%). The simultaneous MRI study demonstrated no high-signal intensity on T2-weighted images in 28 patients (75%). A high–signal-intensity area was observed in 14 patients, and this disappeared 3 months after RT in 8 patients with a negative biopsy. The sensitivity, specificity, and accuracy of MRI tumor response studies at 3 months after radiation therapy were 100%. MRI studies performed after 30 Gy of external-beam irradiation and 3 months after all radiation therapy predicted local tumor control. Similar studies were published by Gong et al.73 and van de Bunt et al.74 Furthermore, MRI is useful in providing accurate target volume definition in brachytherapy treatment planning (Fig. 69.4).75
FIGURE 69.4. A: Magnetic resonance imaging (MRI) at diagnosis showing an enlarged cervical tumor. B: MRI with tandem and ring brachytherapy applicator in place showing dramatic shrinkage of the tumor after concurrent chemotherapy with external beam radiation.