Soft Tissue Sarcoma (Excluding Retroperitoneum)
 INCIDENCE
INCIDENCE
Soft tissue sarcomas (STS) comprise a heterogeneous group of rare malignancies that vary extensively by anatomic location, histology, and biologic behavior. They arise from connective tissues and can occur at any anatomic site. Annually, there are almost 11,000 expected new cases of STS in the United States, and this accounts for approximately 0.7% of all new cancer diagnoses.1 The median age at diagnosis for all STS is 65 years, but incidence varies by histologic subtype.2 For example, embryonal rhabdomyosarcoma is common in children; synovial sarcoma occurs mostly in young adults; and pleomorphic high-grade sarcoma, liposarcoma, and leiomyosarcoma are seen mostly in the elderly.
 ETIOLOGY AND GENETICS
ETIOLOGY AND GENETICS
For the great majority of STS, there is no known etiology. A minority of cases can be attributed to environmental or genetic factors.2,3 Associated environmental factors include radiation exposure; chemical exposures, such as vinyl chloride, dioxin, arsenical pesticides, and phenoxyherbicides; immunosuppression; lymphedema (Stewart-Treves syndrome); and viruses (human immunodeficiency virus, human herpes virus type 8). Certain clinical syndromes are associated with a genetic predisposition for the development of sarcoma. A few examples include Li-Fraumeni syndrome and Werner syndrome, which are associated with the development of STS as well as other malignancies; neurofibromatosis type 1, which is associated with the development of malignant peripheral nerve sheath tumors; and familial adenomatous polyposis (Gardner syndrome), which is associated with the development of abdominal desmoid tumors.
 HISTOLOGIC CLASSIFICATION
HISTOLOGIC CLASSIFICATION
The World Health Organization divides soft tissue tumors into four categories: benign; intermediate, locally aggressive (e.g., desmoid fibromatosis); intermediate, rarely metastasizing (e.g., plexiform fibrohistiocytic tumor); and malignant.2 Further, there are more than 50 histologic subtypes of STS. The most common subtypes include high-grade pleomorphic sarcoma, liposarcoma, leiomyosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumor. Together, these account for about 75% of STS cases.2 Histologic diagnosis is determined largely by tumor cell morphology. Immunohistochemical staining helps refine the diagnosis in many cases, and several of the histologic subtypes have characteristic translocations. Some of these characteristic translocations include: Ewing’s tumor, t(11,22); synovial sarcoma, t(X,18); myxoid liposarcoma, t(12,16); and clear cell sarcoma, t(12,22).2 There are several grading systems. The two most widely used are the U.S. National Cancer Institute (NCI) and the French Federation Nationale des Centres de Lutte Contre le Cancer grading systems, both of which employ a three-tiered system of low, intermediate, and high grade.4 Furthermore, there are certain STS subtypes for which grading is not applicable.2
Given the rarity of STS as well as the numerous histologic subtypes, it is not surprising that diagnoses vary even among STS specialists. Several reports have examined concordance rates for histologic diagnosis or grade among pathologists. Agreement rates between nonspecialists and specialists range from 24% to 68%5–9 Even among sarcoma specialists, concordance rates can vary from 60% to 90%.10,11 For this reason, it is very important to submit diagnostic slides for review by an experienced sarcoma pathologist prior to embarking on definitive treatment.
FIGURE 83.1. Comparison of computed tomography (CT) and magnetic resonance imaging (MRI) axial slices for a synovial sarcoma of the left gluteus medius muscle in a 56-year-old man. A: The CT axial slice shows a vaguely defined soft tissue mass in the left gluteus medius muscle. B: The anatomic definition of the soft tissue sarcoma is seen much more clearly on the corresponding T1 postgadolinium axial slice of the MRI.
 NATURAL HISTORY
NATURAL HISTORY
STS can occur anywhere in the body. The most common site of presentation is an extremity, specifically the thigh. The approximate distribution of STS sites at presentation is extremity, 60% (lower extremity, 45%, upper extremity, 15%); trunk, 15% to 20%; retroperitoneum, 10% to 15%; and head and neck, 8%.12–14,15 Interestingly, certain histologic subtypes have predilections for specific sites. For example, angiosarcoma commonly occurs in the head and neck, desmoid tumors frequently occur in the abdomen associated with Gardner syndrome, and epithelioid carcinoma often involves the hand or forearm.16–18
In the classic reports by Simon and Enneking19 and Enneking et al.,20 the local behavior of STS is well described. STS tends to invade longitudinally along musculoaponeurotic planes. These tumors rarely transgress fascial boundaries or invade bone. As the sarcoma grows, it compresses surrounding normal tissue to form a pseudocapsule, which contains a compression zone and a reactive zone. The latter comprises edema, inflammatory cells, and tumor cells. Furthermore, Simon and Enneking19 have shown that microscopic tumor cells perforate through and extend beyond the pseudocapsule.
Unlike most solid tumors, STS rarely spread to lymph nodes. Three series, each with more than 1,000 consecutive patients with STS, cited only 1.8% to 3.7% of lymph node involvement at the time of initial presentation.21–23 However, there are a few histologic subtypes for which lymph node involvement is more common. Notable lymph node involvement rates have been demonstrated for epithelioid sarcoma (20% to 35%), clear cell sarcoma (10% to 18%), rhabdomyosarcoma (20% to 25%), and cutaneous angiosarcoma (10% to 15%).18,22–26
The American College of Surgeons Patterns of Care Study for adult STS showed that during the years of that study, 23% of patients had metastatic disease at presentation.15 The single most common site of distant metastasis (34%) was the lung; other metastatic sites included bone (24%), liver (16%), brain (3%), and “other” (24%). Two additional studies that examined patterns of distant recurrence (primarily in high-grade STS) also showed the lung to be the most common site for distant spread, reporting 38% to 52% of first recurrences in the lung.13,27 Furthermore, as was the case for lymph node spread, certain histologic subtypes exhibit distinct patterns of recurrence. For example, myxoid liposarcoma has a predilection for spread to the retroperitoneum as well as other extrapulmonary sites. Among reported recurrences, 48% to 71% occur in the retroperitoneum, 20% in extrapulmonary soft tissue, and 15% to 17% in bone.28,29–30 For retroperitoneal STS, the most common site of recurrence is locally in the retroperitoneum.31–34 These tumors also have a predilection for spread to the liver as well as the lung.32–34 Lastly, myxofibrosarcoma exhibits higher local recurrences rates than other STS, sometimes with multiple local recurrences necessitating amputation; conversely, the rate of distant recurrence to the lung for this histology is lower than for other STS types.35–37
 INITIAL EVALUATION
INITIAL EVALUATION
Most STS cases present as a painless mass. When taking a history, one should ask how long the mass has been present, if it has changed in size and at what rate, and if there are any associated local or systemic symptoms. It is also important to ask about potential risk factors for STS, including a history of radiation exposure or a family history of malignancies including STS. On physical examination, one should assess the mass for characteristics such as size, depth, fixation to underlying structures, the presence of overlying skin changes, and potential evidence of neurovascular compromise. On the general examination, one should also note if there are any stigmata of neurofibromatosis, such as neurofibromas or café au lait spots (as there is an association between neurofibromatosis type I and malignant peripheral nerve sheath tumors).3
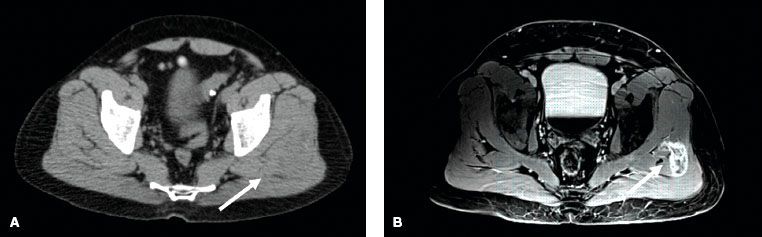
Imaging workup should include evaluation of the primary site as well as sites of potential metastatic spread. For STS of the extremity, trunk, or head and neck, evaluation of the primary tumor with a magnetic resonance imaging (MRI) scan is generally preferred to computed tomography (CT) scanning.38–39,40 The T1-weighted images of the MRI scan provide excellent definition of the anatomic relation between the tumor and adjacent structures. T2-weighted images demonstrate the tumor and associated edema. As peritumoral edema can contain malignant cells, it is crucial to identify this for both radiation and surgical planning.41 Demas et al.39 compared MRI and CT for STS lesions in the extremity and reported that for 23% of cases, the MRI scans showed tumor involvement in muscles that appeared normal on CT scan. On the other hand, a prospective study showed no significant difference between the two imaging modalities when performed for preoperative evaluation.42 Nonetheless, most practitioners believe that MRI is superior to CT for evaluation of soft tissue tumors of the extremity, trunk, and head and neck. Figure 83.1 depicts a case of synovial sarcoma of the left gluteus medius muscle. The mass is difficult to discern on CT imaging (Fig. 83.1A) but very clearly delineated on MRI (Fig. 83.1B). Figure 83.2 is a second comparative example of CT and MRI and shows a leiomyosarcoma of the proximal anterior thigh. Although, the differences between the imaging modalities are not as pronounced as in Figure 83.1, the MRI again provides superior information compared to the CT scan. The MRI demonstrates probable arterial wall invasion, fascial enhancement of the vastus medialis muscle, and definition of tumor spiculations into the adjacent fat and along the biopsy tract. Chest CT scan is recommended to rule out pulmonary metastases for all cases except low-grade tumors or small (<5 cm) high-grade lesions, and even in these latter cases, the author often obtains a baseline chest CT at her institution. Currently, there is no clearly defined role for positron emission tomography (PET) as part of the diagnostic evaluation. However, PET scanning may have potential utility to help distinguish malignant peripheral nerve sheath tumors from benign neurofibromas in patients with neurofibromatosis.43 Lastly, given that myxoid liposarcoma has a predilection for spread to the retroperitoneum, CT of the abdomen and pelvis is recommended as part of the initial evaluation for patients with this histologic subtype.28,29–30
Incisional biopsy or CT-guided core biopsy are the desired diagnostic approaches, and they are preferred to fine-needle aspiration (FNA).44,45 Each of these procedures typically provides enough tissue for adequate pathologic assessment of both histologic subtype and tumor grade. FNA can confirm malignancy for recurrent disease, but typically does not yield enough tissue to establish an initial diagnosis. The diagnostic biopsy should be performed carefully with the subsequent definitive resection in mind.38,46 Tumor cells can potentially seed a biopsy tract or incision, thereby necessitating removal of tracts and skin incisions at the time of surgical resection. It is important that the biopsy approach does not transgress an uninvolved compartment or joint as this would create a situation where a much more radical resection would need to be performed. Consequences of inappropriately placed biopsies can be significant and include the need to perform more complex operations, including amputation, with the potential for subsequent loss of function, local recurrence, and death.46
FIGURE 83.2. Comparison of computed tomography (CT) and magnetic resonance imaging (MRI) axial slices for a high-grade leiomyosarcoma of the proximal anterior thigh in a 53-year-old man. A: The CT axial slice shows a soft tissue mass in the left anterior upper thigh anterior to the superficial femoral artery and vein. B: The T1 postgadolinium image of the MRI at the same level provides superior resolution compared to the CT. It shows a loss of fat plane between the mass and the superficial femoral artery suggestive of arterial invasion (long arrow), linear fascial enhancement overlying the vastus medialis muscle (short arrow), and tumor spiculations into adjacent subcutaneous fat and to the skin along the biopsy tract.
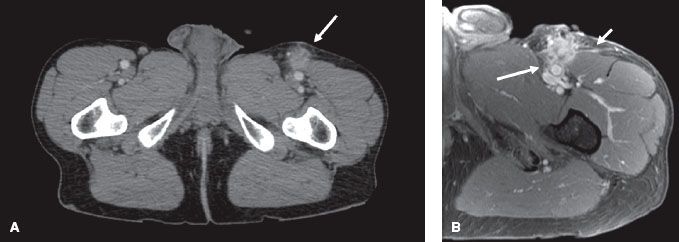
TABLE 83.1 AMERICAN JOINT COMMITTEE ON CANCER TNM STAGE GROUPINGS FOR SOFT TISSUE SARCOMAS
 STAGING
STAGING
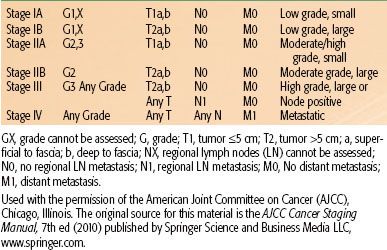
The American Joint Committee on Cancer (AJCC) published the seventh edition of the TNM (tumor, node, metastasis) staging manual in 2010.47 The significant factors related to staging of STS include grade (1 to 3), tumor size (≤5 cm vs. >5 cm), location superficial or deep to fascia, and presence of lymph node or distant organ involvement. Stage groupings are shown in Table 83.1. The AJCC staging system for STS does not account for histologic subtype or tumor site, nor does it stratify for tumor size >5 cm. This is unfortunate, given that all of these factors are predictive of survival, but understandable given the complexities of staging systems.
 PROGNOSTIC FACTORS FOR SURVIVAL AND LOCAL RECURRENCE
PROGNOSTIC FACTORS FOR SURVIVAL AND LOCAL RECURRENCE
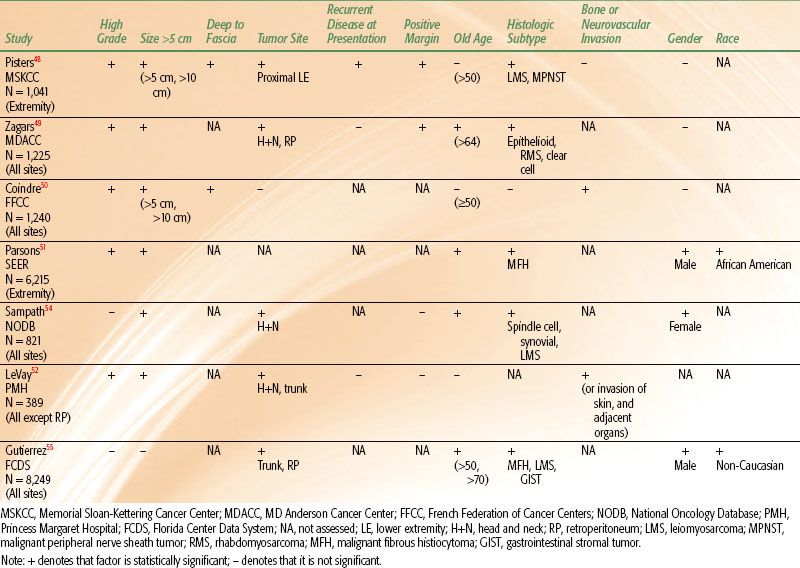
Many reports have evaluated patient and tumor characteristics to determine prognostic factors for disease-free survival (DFS) or overall survival (OS) and local recurrence (LR). The most powerful predictor for DFS and OS is the AJCC TNM stage of the tumor. Five-year DFS rates for stages I, II, and III STS are 86%, 72%, and 52%, respectively.47 The staging system incorporates several variables, the two most important of which are grade and tumor size. It is also valuable to consider predictive factors individually. Results of multivariate analyses for DFS from several large studies are shown in Table 83.2. Although there are differences among series, several independent prognostic factors are consistently demonstrated. The single most important individual prognostic factor for lower survival rates is high grade.48–53 Five-year DFS rates range from 44% to 67% for high-grade tumors compared to 90% to 100% for low-grade tumors.48–50,53 Other significant predictors for DFS include tumor size, depth, and site. Tumors >5 cm are associated with 5-year DFS rates of about 55% to 70% compared to rates of about 78% to 100% for tumors <5 cm.48,50,53 DFS rates for tumors >10 cm are even lower and range from 33% to 60%.48,50 Five-year DFS rates for tumors that are deep to the fascia range from 58% to 70%, whereas those for tumors superficial to fascia are 81% to 92%.48,50 Furthermore, patients with tumors located in the head and neck or retroperitoneum have lower survival rates than those with tumors located in the extremity or superficial trunk.49,52,54,55 Reports vary regarding the impact of histologic subtype on DFS, but several cite leiomyosarcoma and malignant peripheral nerve sheath tumor as adverse prognostic factors.48,54–56 Older age at presentation, positive resection margins, bone or neurovascular invasion, gender, and race all show mixed results regarding their predictive value for DFS (Table 83.2). As stated earlier, lymph node involvement for STS is rare; but if present, it is an adverse prognostic factor.21–23
Consistently demonstrated significant predictors for LR include positive margins of resection, presentation with locally recurrent disease, older age, and head and neck or retroperitoneal location. Rates of LR for tumors resected with positive margins range from 28% to 56% compared to 0% to 20% for those with negative margins.48,49,57–63 Patients who present with locally recurrent disease are at higher risk for LR (25% to 47%) than those who present with primary disease (11% to 21%).48,58,61 Age has been analyzed with varying cutoff values including >50 years, >64 years, and as a continuous variable. Repeatedly, older age has been associated with higher LR rates.48,49,52,54
TABLE 83.2 SIGNIFICANT ADVERSE PROGNOSTIC FACTORS FOR DISEASE SPECIFIC SURVIVAL OR OVERALL SURVIVAL ON MULTIVARIATE ANALYSES FROM SEVERAL LARGE PATIENT SERIES
 MANAGEMENT AND OUTCOME FOR SOFT TISSUE SARCOMAS OF EXTREMITY AND TRUNK
MANAGEMENT AND OUTCOME FOR SOFT TISSUE SARCOMAS OF EXTREMITY AND TRUNK
Because of the rarity of STS and the numerous forms in which it can present with respect to tumor histology, site, and size, there are many nuances related to optimal management. Furthermore, delivery of treatment requires a multimodality team that includes experienced pathologists; radiologists; surgeons from the disciplines of surgical oncology, orthopedics, and reconstructive surgery; radiation oncologists; medical oncologists; nurses; physical therapists; and social workers. Treatment goals include complete eradication of tumor with optimal function preservation and minimal treatment-related toxicities. Execution of these goals is complex, and, for this reason, STS is best treated by an experienced team at a specialized sarcoma center. Reports from Sweden, the United Kingdom, and the United States have shown inferior quality of treatment delivery and outcome for STS treated outside of specialized centers.64–66
Surgery
In almost all cases, appropriate surgical resection is a prerequisite for curative treatment of STS. A range of surgical procedures has been employed for the treatment of STS with varying levels of success. These procedures include marginal resection or excisional biopsy, wide resection, and radical resection or amputation. A marginal resection refers to simple removal of the tumor with its pseudocapsule. This is also often described as a “shell-out,” and this is the procedure commonly performed when the diagnosis of STS is not suspected. LR rates after marginal resection range from 42% to 93%.12,20,67–70 This is not surprising as it is known that microscopic tumor cells can extend beyond the pseudocapsule and up to several centimeters beyond palpable gross tumor.19,41 Marginal resection is not an appropriate treatment.
At the other end of the spectrum is radical resection, which involves removal of all of the muscles and neurovascular structures within the compartment where the tumor resides or amputation. Reported LR rates after radical resection are much lower and range from 0% to 18%.12,20,59,67–69 These LR rates are acceptable, but the cost of loss of limb (or loss of an entire compartment) is high. Amputation was a common procedure for STS of the extremities up to the 1970s. The intermediate procedure is a wide resection. Wide resection is also described as conservative surgery (CS), limb-sparing surgery, or function-sparing surgery. It involves en bloc removal of tumor with a rim of normal tissue varying in width from about 1 cm to several centimeters depending on anatomic constraints. This procedure preserves good function (limb salvage) but as a treatment by itself is usually associated with moderately high LR rates, ranging from 25% to 60%.12,20,67–69 Wide resection/CS combined with pre- or postoperative radiation therapy (RT) is the current standard of care for most high-grade STS.
Surgeons should attempt to attain negative margins at the time of definitive resection. As previously stated, the presence of positive margins is consistently associated with increased LR rates even when RT is used.48,49,52,57–63,71 Because STS is so rare in comparison to benign soft tissue lesions, the initial procedure performed for a STS is often an unplanned excision (shell-out) with resulting positive margins. It is important to perform a definitive re-excision in these situations, if possible, as the likelihood of finding significant residual disease is on the order of 24% to 63%.8,72,73–75,76–79 As part of the re-resection, incisions, biopsy tracts, drain sites, and any tissues contaminated by the first surgery need to be removed en bloc along with tumor-bed margins. Unfortunately, this often results in a greater scope of surgery and increased functional deficit than if an initial planned excision had been performed by an experienced oncologic surgeon.46 Lastly, although the goal of resection is to attain negative margins, if this would require debilitating surgery, such as resection of a nerve, vessel, or bone, a function-sparing approach with a planned positive margin is sometimes accepted. These decisions are best handled by experienced sarcoma surgeons. Gerrand et al.60 compared LR rates resulting from procedures with planned positive margins (abutting critical structures) to those of procedures with unplanned (unexpected) positive margins. They found a 4% LR rate for the former compared with a 32% to 38% LR rate for the latter.
TABLE 83.3 RANDOMIZED CONTROLLED TRIALS INCLUDING RADIATION THERAPY FOR SOFT TISSUE SARCOMAS OF THE EXTREMITIES AND TRUNK
Conservative Surgery and Radiation Therapy
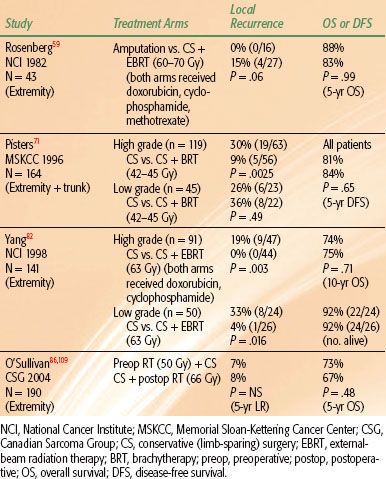
Three sentinel randomized trials have been performed and have established RT combined with CS as the standard management for most (high-grade) STS of the extremities and trunk (Table 83.3). The first of these trials was conducted by Rosenberg et al.59 at the NCI. Patients with high-grade STS of the extremity were randomized to amputation or to CS and postoperative external beam RT (60 to 70 Gy). Patients in both treatment arms received postoperative doxorubicin, cyclophosphamide, and methotrexate; in the RT arm, chemotherapy was started 3 days prior to RT and continued concurrently with RT. LR rates were 0% (0 of 16) and 15% (4 of 27) for patients treated with amputation and with CS and RT, respectively (P = .06). There was no significant difference in survival rates. This trial was instrumental in setting a new standard for limb-sparing local management of STS. Amputations are now performed sparingly and in <15% of cases.80,81
The next question posed was whether adjuvant RT is necessary. Two more hallmark trials randomized patients between CS alone and CS plus RT.71,82 Both of these trials showed improved local control with the addition of RT. At the NCI, patients with high-grade STS of the extremity were randomized to treatment with CS and postoperative chemotherapy (doxorubicin and cyclophosphamide) with or without concurrent external-beam RT (63 Gy). Patients with low-grade tumors were randomized to CS with or without postoperative external-beam RT (63 Gy). LR rates for the high-grade tumors were 20% (9 of 44) for CS and chemotherapy compared to 0% (0 of 47) for CS, chemotherapy, and RT (P = .003). For the low-grade tumors, LR rates were 33% (8 of 24) for CS alone and 4% (1 of 26) for CS and RT (P = .016). There were no significant differences in survival rates between the two groups. At Memorial Sloan-Kettering Cancer Center (MSKCC), patients with STS of the extremity and trunk were randomized to treatment with CS alone or CS plus adjuvant brachytherapy (BRT).71 BRT catheters were sewn into the tumor bed in a parallel array with 1-cm spacing between catheters and extension of catheters 1.5 to 2.0 cm beyond the tumor bed. Iridium-192 was loaded into the catheters and a dose of 42 to 45 Gy was delivered over 4 to 6 days. LR rates for high-grade tumors were 30% (19 of 63) for CS alone compared with 9% (5 of 56) for CS plus BRT (P = .0025). No difference in LR rates by treatment was seen for low-grade tumors; rates were 26% (6 of 23) and 36% (8 of 22) for patients treated with CS alone and CS plus BRT, respectively. As in the two prior trials, there were no significant differences in survival outcomes. Given that BRT did not improve local control for low-grade tumors in this trial, BRT is not recommended for low-grade STS. Furthermore, for high-grade tumors treated with BRT, LR rates are higher in the setting of positive margins.83 Therefore, BRT as monotherapy is only recommended for high-grade tumors resected with negative margins.84 (In the setting of positive resection margins, a combination of external-beam RT and BRT or external-beam RT alone is preferred.) These two randomized trials have established the role for RT combined with CS for the management of high-grade (and, in select cases, low-grade) STS. In modern series, local control following CS and RT is excellent, with most reported LR rates being <15%.53,58,63,85–91
It is important to note that the current treatment recommendation for most low-grade STS of the extremity and trunk is wide excision alone.92 As long as negative margins are obtained, LR rates are expected to be well under 20%. Relative indications for RT in the setting of low-grade tumors include situations of positive resection margins, locally recurrent disease following initial wide excision, and tumor location that would not be amenable to subsequent salvage surgery.
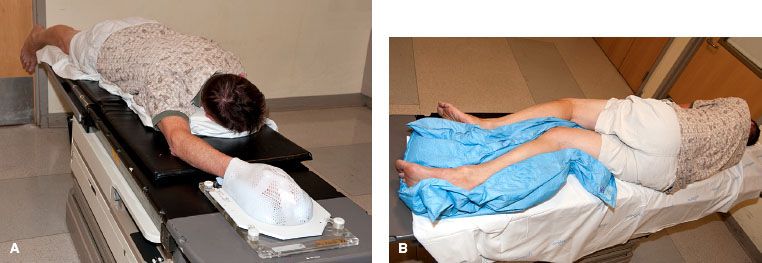
FIGURE 83.3. Examples of patient positions for treatment of upper and lower extremity soft tissue sarcoma (STS). Patients are immobilized in custom casts. A: The “swimmer position” is often an ideal position for treating STS of the hand, forearm, or distal upper arm. B: This is a right decubitus position to treat a STS in the right posterior or anterior distal thigh or lower leg. The right leg is placed directly on the table. The left leg is flexed at the knee, positioned anterior to the right leg, and the legs are separated as much as possible.
Radiation Therapy
Positioning the Patient
The first step of RT planning is to determine the appropriate positioning of the patient. This can be challenging, and sometimes simulation needs to be performed in a few different positions to ascertain the optimal arrangement. A limb should be positioned to allow treatment with as many potential beam angles as possible. In general, this requires that the limb is positioned as far away from the trunk (for upper extremities) or from the opposite limb (for lower extremities) as possible. Suitable positions for upper extremity lesions often include abduction of the arm away from the body with supination or pronation of the arm determined by the location of the tumor. Another good approach for upper extremity lesions is the “swimmer position,” whereby the patient is prone with the arm extended above the head (Fig. 83.3A). This position enables an almost 360-degree approach for all but proximal lesions close to the head. For lower extremity lesions, there are several potential positions. Tumors in the medial compartments are often treated with the majority of the beams in an anterior-posterior/posterior-anterior–like orientation and therefore positioning the patient supine with the legs spread apart often suffices. For proximal thigh lesions, one or both legs are often abducted and flexed in the “frog-leg position” to enable reduction of skin folds in the inguinal region, optimal sparing of the perineum and genitalia, and maximal separation from the contralateral leg. Tumors in the true anterior or posterior compartments are often the most challenging. Sometimes the supine position and oblique fields will be feasible, but, for most of these cases, the author’s approach is to place the patient in a decubitus position (Fig. 83.3B). The author places the patient in the right-sided decubitus position for right-sided tumors (and vice versa), as she finds it is more stable to have the treated leg directly on the table. The untreated leg is flexed at the knee and placed either posterior or anterior to the treated leg, and the legs are separated as much as possible. For men with proximal thigh lesions, the author typically places the genitalia in netting, which is pulled to the contralateral side. Most of time, the genitalia can be positioned such that they will be out of the path of any beams, but the dose delivered from internal scatter cannot be avoided. For this reason, the author counsels all men with treatment fields close to the genitalia to consider sperm banking if they wish to preserve fertility. Similarly, the author recommends fertility consultation for women with treatment fields close to the ovaries. The author also typically calculates and documents doses delivered to the testicles or ovaries in appropriate situations. Patients with an extremity STS that is to be treated with intensity-modulated RT (IMRT) are often positioned in a more neutral supine posture.93 This is possible because of the multiple beam angles used in IMRT planning. Once the position is determined, the patient is immobilized in a reproducible fashion. A custom cast is highly recommended for almost all scenarios.
Target Volumes and Treatment Fields
Preoperative Radiation Therapy
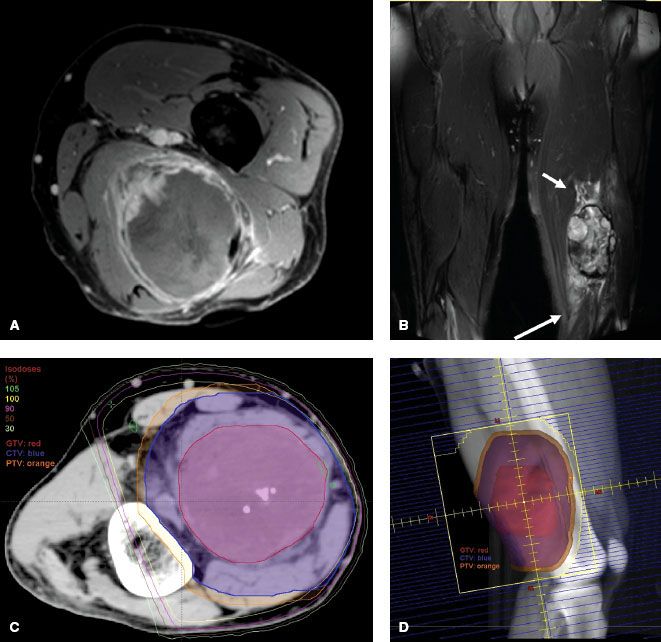
Definitions of appropriate treatment volumes for STS of the extremities and trunk have not been formally tested. Historically, large longitudinal margins of at least 5 cm and sometimes >10 cm have been used with good success. The Radiation Therapy Oncology Group (RTOG) Sarcoma Working Group reported a consensus for appropriate target volumes for preoperative RT and this is described as follows.94 The gross tumor volume (GTV) is defined as the gross tumor delineated by the T1 postgadolinium MRI. Fusion of the diagnostic MRI and planning CT for optimal target definition is strongly encouraged. Clinical target volume (CTV) is defined as the GTV plus 3-cm margins in the longitudinal directions and 1.5-cm margins radially. These margins can be truncated if they extend beyond the compartment or into an intact fascial barrier, bone, or skin. Peritumoral edema on T2 MRI will often be included within the CTV as defined above. If the edema is more extensive and appears suspicious, the CTV can be enlarged to include it at the discretion of the radiation oncologist. Of note, more clarity is needed to determine in which situations peritumoral edema should be included in the CTV. One report showed the presence of sarcoma cells beyond the gross tumor in 10 of 15 patients. The location of the cells varied from 1 to 4 cm beyond the tumor but did not correlate with the location or extent of peritumoral edema on MRI.41 It is reasonable to try to include the edema in the CTV, but if this would require a significant increase in the treatment field beyond the RTOG consensus guidelines, it becomes a judgment call. The planning target volume (PTV) definition is not specified in the consensus statement, but typically it is defined as the CTV plus 5 to 10 mm. Application of the GTV and CTV definitions of the RTOG consensus group results in treatment planning fields very similar to the historical standard of 5-cm margins and 2.5- to 3-cm margins from GTV to the field edge in the longitudinal and radial dimensions, respectively. Figure 83.4 depicts MRI, dosimetry, and treatment field images for a STS of the left posterior thigh that was treated with three-dimensional (3D) conformal preoperative RT.
Several series using treatment volumes similar to those described above have reported excellent local control rates. Kim et al.88 reported a series of 56 patients treated using target volume definitions very similar to the consensus recommendations and described a local control rate of 88.5%. The Canadian Sarcoma Group’s randomized trial of preoperative versus postoperative RT also employed similar preoperative treatment fields (except that the CTV expansion in the longitudinal direction was typically 4 cm rather than 3 cm) and reported a 5-year actuarial local control rate of 93%.86 Lastly, a review of 768 patients treated with preoperative and postoperative RT at Princess Margaret Hospital between 1990 and 2006 demonstrated a local control rate of 92%. Among the 60 recurrences, 82% occurred within the treatment fields.87 As a group, these three publications provide firm support that the current guidelines produce treatment fields that are sufficiently large.
FIGURE 83.4. An unclassified pleomorphic high-grade sarcoma of the posterior left thigh in a 61-year-old man that was treated with preoperative three-dimensional-conformal radiation therapy (50 Gy) using opposed oblique fields. A: T1 postgadolinium image of the magnetic resonance imaging (MRI) scan. B: T2 image of the MRI scan showing peritumoral edema (arrows). C: Axial slice of the graphic plan showing the gross tumor volume (red), clinical target volume (blue), planning target volume (orange), and covering isodose lines. D: Digital reconstructed radiograph showing the treatment field and target volumes.