(1)
Glasgow Royal Infirmary, Glasgow, UK
4.1 Introduction
Type 2 diabetes is becoming a common problem in young obese people and, in some parts of the world, is as common as type 1 diabetes in teenagers and young adults. However, patients that are included in the Phase III trials of new antidiabetic drugs tend to be middle-aged and elderly and, at this point in time, there is little information on the effects of sodium glucose cotransporter 2 (SGLT2) inhibitors in patients under 19 or over 75 years of age. Similarly, until there is much more safety data on SGLT2 inhibitors, these drugs are untested in pregnancy and gestational diabetes or in patients with serious liver disease. In pregnancy, the renal threshold for glucose reduces (i.e., glucose spills over into the urine at a lower blood glucose concentration), and so different clinical effects can be anticipated when compared to the nonpregnant population. Thus, this chapter will omit these patients and focus on the effects of SGLT2 inhibitors in four specific groups of patients where there is published information on efficacy, safety, or both. This includes:
Patients with cardiovascular disease (CVD)
Patients with chronic kidney disease (CKD)
Older patients with type 2 diabetes
Patients with type 1 diabetes
4.2 SGLT2 Inhibitors in Patients with Cardiovascular Disease
SGLT2 inhibitors potentially have multiple effects on cardiovascular risk markers (Table 4.1) [1]. These include improved glycemia, reduced blood pressure, and reduced body weight, which are all potentially favorable effects on cardiovascular risk factors. They also cause slight but significant effects on lipids, with increases in low-density lipoprotein (LDL) cholesterol, which might potentially increase cardiovascular events.
Table 4.1
Potential effects of SGLT2 inhibitors on markers of cardiovascular risk
Potentially beneficial | Potentially harmful |
|---|---|
Improved glycemia Reduced blood pressure Reduced albuminuria Reduced arterial stiffness Reduced sympathetic nervous activity Reduced weight Reduced visceral adiposity Reduced oxidative stress Reduced uric acid Reduced triglycerides Increased HDL cholesterol | Increased LDL cholesterol |
The Food and Drug Administration (FDA) in the United States and European Medicines Evaluation Agency (EMEA) in Europe closely scrutinize the cardiovascular effects of all new treatments for type 2 diabetes. New antidiabetic drugs have to demonstrate that they are safe and do not increase the risk of cardiovascular events. Patients with higher cardiovascular risk are deliberately included in large Phase III trials of new antidiabetic drugs, and atherosclerotic events such as myocardial infarction, stroke, cardiovascular death, or hospitalization for unstable angina are blindly adjudicated. A randomized, controlled cardiovascular safety trial is usually mandated, although on most occasions the drug will be approved on the basis of the safety in the Phase III trials before the randomized controlled trial (RCT) is completed.
4.2.1 EMPA-REG OUTCOME
EMPA-REG OUTCOME was a large cardiovascular safety study in 7020 people with type 2 diabetes and existing CVD [2]. It compared empagliflozin 10 mg, empagliflozin 25 mg, and placebo in addition to usual standards of care, and nearly half of the participants were on insulin. The first patient was enrolled in 2010 and the study was completed in 2015. Forty-seven percent (47 %) of the patients had a prior myocardial infarction, 25 % had a previous coronary artery bypass graft (CABG), 23 % had a history of stroke, 20 % had peripheral arterial disease, and 10 % had heart failure. Additionally, 26 % of the patients had a baseline eGFR (estimated glomerular filtration rate) between 30 and 60 mL/min/1.73 m2. In other words, this was group of subjects at very high-risk of cardiovascular events.
The results were remarkable, as empagliflozin was found to be superior to placebo in reducing major adverse coronary events (defined in the study as cardiovascular death, nonfatal myocardial infarction, nonfatal stoke) and reduced total mortality by 32 % (Fig. 4.1) [3]. The effects were the same for both doses (10 and 25 mg) of empagliflozin. Significantly, hospitalization for heart failure was reduced by one-third [3]. With regard to side effects and safety, there was the expected increase in genital infections and a slight increase in urosepsis in patients treated with empagliflozin, but there was no increase in hypoglycemia, fractures, or ketoacidosis.
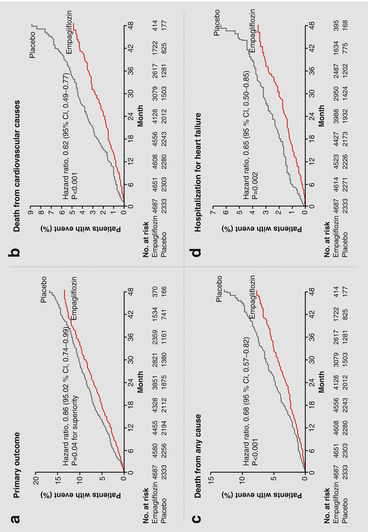

Figure 4.1
Cardiovascular outcomes and death compared to placebo in the EMPA-REG OUTCOME trial. Reductions in the (a) primary outcome, (b) cardiovascular death, (c) total mortality, and (d) hospitalization for heart failure with empagliflozin (Reproduced with permission from Zinman et al. [3] ©Massachusetts Medical Society)
Several mechanisms for the cardiovascular risk benefit can be postulated, including reductions in blood pressure, weight loss, and diuresis; it seems likely that all of these mechanisms contribute, rather than it being a single factor. It will not be possible to say if this benefit is unique to empagliflozin or if this is a class effect shared with other SGLT2 inhibitors until cardiovascular safety trials with the other SGLT2 inhibitors are completed. The impressive reduction in total mortality seen with empagliflozin in the EMPA-REG OUTCOME trial will lead to a change in the management of patients who have existing CVD and are uncontrolled on insulin therapy. Thus, initially, the increased use of empagliflozin should be focused on this challenging patient group.
4.2.2 Cardiovascular Trials with Other SGLT2 Inhibitors
Dapagliflozin was approved by the FDA and EMEA on the basis of cardiovascular safety as part of a Phase III trial program [4]. DECLARE-TIMI 58 is a large RCT comparing dapagliflozin 10 mg with placebo in around 17,000 patients aged >40 years and at high risk for cardiovascular events, with an estimated completion date of 2019. As with EMPA-REG OUTCOME, the primary outcome is a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. Secondary outcomes include hospitalization for heart failure and all-cause mortality.
To approve canagliflozin, the FDA requested data from the ongoing CANVAS trial, an RCT comparing canagliflozin 100 and 300 mg with placebo in patients with either existing CVD or two or more cardiovascular risk factors [5]. Two-thirds of the patients in CANVAS have existing CVD and the double-blind study has an estimated completion date of 2017. A second RCT has been established with canagliflozin called CANVAS-R. It will recruit a similar group of patients with a primary outcome of measuring the progression of albuminuria; the cardiovascular results from CANVAS and CANVAS-R will be combined to satisfy FDA postmarketing requirements for canagliflozin.
Ertugliflozin is a newer SGLT2 inhibitor and is currently in Phase III of development. Recruitment has been completed for a cardiovascular outcomes trial comparing ertugliflozin 5 mg, 15 mg, and placebo in patients with established vascular complications. It is estimated that the trial will run for between 5 and 7 years. The cardiovascular outcome trials involving SGLT2 inhibitors are compared in Table 4.2.
Table 4.2
Cardiovascular outcomes trials with SGLT2 inhibitors
Study | Comparators | Inclusion criteria | Primary endpoint | Estimated completion |
|---|---|---|---|---|
EMPA-REG OUTCOME | Empagliflozin 10 and 25 mg vs. placebo | Established CVD | CV death, non-fatal MI, non-fatal stroke | Completed 2015 |
CANVAS | Canagliflozin 100, 300 mg vs. placebo | Established CVD or high CV risk | CV death, non-fatal MI, non-fatal stroke | 2017 |
DECLARE TIMI 58 | Dapagliflozin 10 mg vs. placebo | High CV risk | CV death, non-fatal MI, non-fatal ischemic stroke | 2019 |
Ertugliflozin CVOT | Ertugliflozin 5 and 15 mg vs. placebo | Established CV disease | CV death, non-fatal MI, non-fatal stroke | 2020 |
4.3 SGLT2 Inhibitors in Patients with Chronic Kidney Disease
The mode of action of SGLT2 inhibitors reduces the reabsorption of glucose in the kidney, increasing glycosuria. Thus, it can be hypothesized that SGLT2 inhibitors would be less effective in reducing glycated hemoglobin (HbA1c) levels in patients who have renal impairment, where urinary glucose excretion is reduced by up to 50 %. This has been examined by performing dedicated studies in patients with CKD, and by examining all patients with CKD in the Phase III development program (e.g., as done with canagliflozin). All three of the currently available SGLT2 inhibitors have been studied in groups of patients with CKD.
Dapagliflozin (at doses of 5 and 10 mg) was compared with placebo in 252 patients with stage 3 CKD (estimated glomerular filtration rate [GFR] 30–59 mL/min/1.73 m2) [6]. Serum creatinine increased at week 1 and then did not change after a further 2 years of treatment. This is similar to the changes in creatinine that are seen in people with normal renal function. Although blood pressure and weight both reduced with dapagliflozin, there was no significant reduction in HbA1c compared to placebo in that study [6]. Other studies analyzing dapagliflozin in patients with CKD are in progress.
Canagliflozin (100 and 300 mg) was compared with placebo in 269 patients with a subset of stage 3 CKD (eGFR 30–50 mL/min/1.73 m2). As well as reducing weight and blood pressure, a significant reduction in HbA1c was observed at 26 weeks and at 1 year [7]. Urinary tract infections and osmotic diuresis-related adverse events were more common with canagliflozin 300 mg. Decreases in eGFR were observed with both doses of canagliflozin, and both doses reduced urine albumin–creatinine ratios vs. placebo.
THE EMPA-REG RENAL trial was a comprehensive trial of empagliflozin in patients with three different stages of CKD [8]. Empagliflozin was studied in patients with stage 2 CKD (eGFR 60–89 mL/min/1.73 m2) comparing empagliflozin 10 mg, 25 mg, and placebo, and in 374 patients with stage 3 CKD (eGFR 30–59 mL/min/1.73 m2) comparing empagliflozin 25 mg and placebo. HbA1c was significantly reduced in both of these groups of patients when compared to placebo, with reductions in weight and blood pressure. A total of 74 patients with CKD stage 4 (eGFR 15–29 mL/min/1.73 m2) were studied with empagliflozin and placebo, and in these patients empagliflozin was not effective at reducing HbA1c.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree