(1)
Glasgow Royal Infirmary, Glasgow, UK
3.1 Introduction
Evidence-based clinical practice guidelines are systematically developed statements intended to assist practitioners and patients about making and understanding care decisions for specific clinical circumstances. The evidence base should be obtained using an unbiased and transparent process of systematically reviewing and appraising published clinical research, which is then synthesized into recommendations for clinical practice. There is a hierarchy of evidence involved, from meta-analysis or systematic reviews of randomized controlled trials (RCTs), to case-controlled or cohort studies, to expert opinion. Evidence-based guidelines have largely replaced consensus statements, which typically involves a group of experts meeting and producing a series of recommendations based on the consensus of the group at that time. However, these can be prone to bias depending on the opinions of those involved in the process, and often would be based on a limited literature review which might miss key publications, especially if the results are negative.
Guidelines for the management of type 2 diabetes exist at local, national, and international levels and some examples of these are given in Table 3.1 [1–13]. These usually include glycemic targets for HbA1c and recommendations on the therapeutic options that can be used to reach these targets. Pharmacological monotherapy is started after a period of lifestyle adjustment, including changes to the diet and increases in physical activity. Most guidelines recommend first-line therapy with metformin based on the results of the United Kingdom Prospective Diabetes Study (UKPDS) plus the slight reduction in weight that is obtained with metformin [14]. Thereafter, if targets are not met then the choice of second-line therapy varies from guideline to guideline.
Table 3.1
A selection of major treatment guidelines for the management of type 2 diabetes
Region | Guideline |
|---|---|
International | International Diabetes Federation (IDF): Global Guideline for Type 2 Diabetes (2012) [1] |
National | |
Canada | Canadian Diabetes Association (CDA): Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada (2013) [4] |
England, Wales, and Northern Ireland | National Institute for Health and Care Excellence (NICE): Type 2 Diabetes in Adults: Management (2015) [5] |
Scotland | Scottish Intercollegiate Guidelines Network (SIGN): Management of Diabetes (2011) [6] |
USA | |
Spain | Working Group for Consensus Documents and Clinical Guidelines of the Spanish Diabetes Society: Recommendations for the Pharmacologic Treatment of Hyperglycemia in Type 2 Diabetes. Consensus Document (2011) [9] |
Germany | German National Disease Management Guideline on the Treatment of Type 2 Diabetes (2013) [10] |
Japan | Japan Diabetes Society: Evidence-based Practice Guidelines for the Treatment of Diabetes in Japan (2013) [11] |
India | Indian Council of Medical Research: Guidelines for Management of Type 2 Diabetes. Pharmacological Treatment of Diabetes (2005) [12] |
Brazil | Brazilian Diabetes Society: Algorithm for the Treatment of Type 2 Diabetes. Position Statement (2010) [13] |
3.2 International Diabetes Federation Guidelines
The 2012 International Diabetes Federation (IDF) global guideline for type 2 diabetes focuses particularly on cost, availability, and side effects of drugs [1]. The IDF guidelines recommend that metformin should be used as first-line therapy, with sulfonylureas as second-line and insulin third. More recent drug classes such as dipeptidyl peptidase-4 (DPP-4) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists are mentioned as second-line alternatives, based on the fact that the cost of these drugs is greater than metformin and sulfonylureas because there are no generic formulations and they are not available for clinical use in every country. Sodium glucose cotransporter 2 (SGLT2) inhibitors are not included in the IDF guidelines, as no drugs in this class had been approved when the guideline was developed.
3.3 Joint American Diabetes Association and European Association for the Study of Diabetes Position Statement
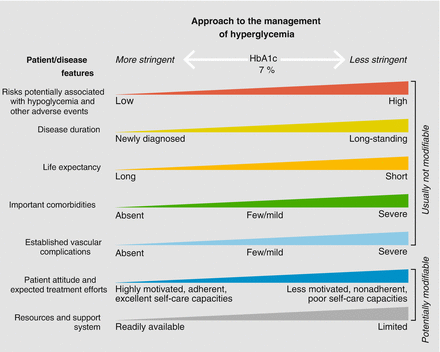
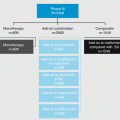
In 2012, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) published a joint position statement on the management of hyperglycemia in patients with type 2 diabetes [2]. This was published in response to the increasing number of antidiabetic drugs being developed and was intended to be more evidence-based than previous joint statements, which had primarily been consensus reports. An important emphasis was placed on taking a patient-centered approach to making clinical decisions and aiming to provide care that is respectful of and responsive to individual patient preferences, needs, and values. Less stringent glycemic targets were suggested, depending on factors such as risks of hypoglycemia, disease duration, life expectancy, important comorbidities, and established vascular complications. Patient attitude, expected treatment efforts, resources, and support system are also to be taken into consideration (Fig. 3.1).
Considerations for the choice of glucose-lowering agent include age, weight, gender, racial, and genetic differences, as well as serious comorbidities such as coronary artery disease, heart failure, chronic kidney disease, and liver dysfunction. Again, as no SGLT2 inhibitors had been approved, they were not included as a treatment option in the 2012 ADA/EASD position statement.
3.3.1 Update to the Joint ADA/EASD Position Statement
The position statement was updated in 2015 to reflect new data from recent clinical trials and intended as an addendum to the 2012 full version [3]. In the update, SGLT2 inhibitors were described as a major change in antidiabetic treatment and that because the mode of action was independent of insulin action, could be used at any stage of type 2 diabetes, including when insulin secretion had waned significantly. Potential advantages of using SGLT2 inhibitors were noted and included lower risk of hypoglycemia, potential weight loss, and lowering of systolic and diastolic blood pressure, as well as potential disadvantages such as genitourinary infections, volume depletion, an increase in low density lipoprotein (LDL) cholesterol, and a transient increase in creatinine. The cost of SGLT2 therapy was described as “high” (Table 3.2).
Table 3.2
Properties of SGLT2 inhibitors according to 2015 American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) position statement
Class | Compounds | Cellular mechanisms | Primary physiological actions | Advantages
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|