and Rachel Livingstone2
(1)
Glasgow Royal Infirmary, Glasgow, UK
(2)
Specialty Trainee, Royal Alexandria Hospital, Paisley, UK
2.1 Mode of Action and Clinical Pharmacology
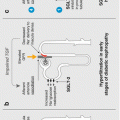
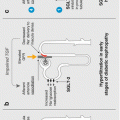
Sodium-glucose cotransporter-2 (SGLT2) inhibitors have a novel therapeutic action when compared with the many drugs available to the prescriber to treat type 2 diabetes. The pharmacological action of this drug class is in the kidneys, which play an important role in glucose homeostasis by filtering and reabsorbing glucose in the proximal tubules. On a daily basis, the kidney filters approximately 180 g of glucose. The majority of filtered plasma glucose (80–90 %) is reabsorbed in the early proximal tubule by the high capacity, low affinity SGLT2 [1]. The other 10–20 % is reabsorbed by the low capacity but high-affinity SGLT1 in the more distal portion of the proximal tubule (Fig. 2.1) [2]. This is achieved by cotransporting glucose with sodium via Na+/K+–adenosine triphosphatase pumps. SGLT2 is selectively expressed in the kidney, whereas SGLT1 is also expressed in the gastrointestinal tract where it has a role in the absorption of glucose and galactose [3]. In healthy individuals, virtually all glucose is reabsorbed and the urine is free from glucose.
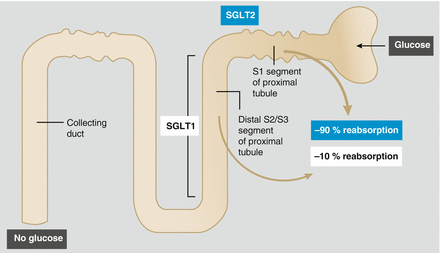

Figure 2.1
Normal glucose homeostasis within the kidney. In normal renal glucose homeostasis approximately 90 % of filtered glucose is reabsorbed in the proximal tubule by SGLT2. In patients with poorly controlled diabetes the renal threshold for glucose reabsorption can be exceeded resulting in glycosuria. SGLT2 sodium-glucose cotransporter 2 (Reproduced with permission from Chao and Henry [2] ©Nature)
The filtration and reabsorption of glucose are directly proportional to plasma glucose levels. In animal models of type 2 diabetes, there is increased expression of SGLT1 and SGLT2 mRNA due to prolonged hyperglycemia and, therefore, enhanced activity. This causes alterations to glucose handling by the kidneys and results in the maximum threshold for glucose reabsorption to be increased, and ultimately conserves glucose and exacerbates hyperglycemia [4].
They act by inhibiting SGLT2 in the kidneys, reducing the reabsorption of glucose in the proximal convoluted tubule and increasing glucose excretion in the urine (Fig. 2.2) [2]. The glucose excreted in the urine equates to a net loss of 200–300 kcal/day and weight reduction is a favorable secondary effect. SGLT2 inhibitors have many benefits over alternative therapeutic options and act independently of pancreatic β cell function, which deteriorates over time, and therefore there should be no loss of potency with long-term use. Additionally, SGLT2 inhibition does not interfere with endogenous insulin production or endogenous glucose production in response to hypoglycemia and therefore does not increase the risk of hypoglycemia.
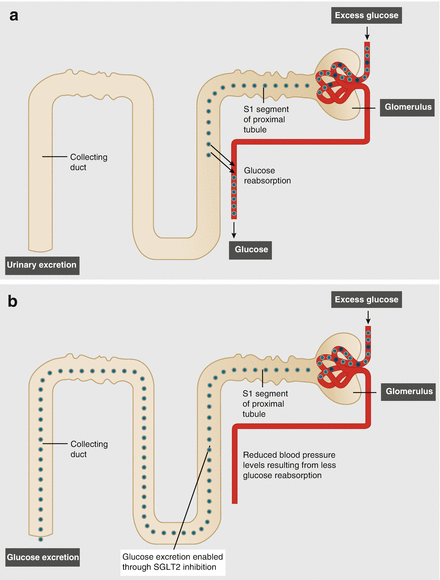

Figure 2.2
(a, b) Effect of SGLT2 inhibitors on glucose homeostasis. Blockade of SGLT2 allows for more of the filtered glucose to be excreted in urine increasing the glycosuria with resultant reduction in blood glucose. SGLT2 sodium-glucose cotransporter 2 (Adapted with permission from Chao and Henry [2] ©Nature)
2.2 Drug Development and Major Clinical Trials
2.2.1 Phlorizin
Early investigations into renal glucose handling were carried out on phlorizin, which is found in the root bark of fruit trees. It is a naturally occurring competitive inhibitor of SGLT1 and SGLT2 but has greater affinity for the latter. In the 1980s, a rat model of diabetes demonstrated that phlorizin-induced glycosuria was associated with normalization of hyperglycemia without inducing hypoglycemia. Phlorizin is metabolized to phloretin in the gastrointestinal tract by glucosidase, and therefore has poor systemic bioavailability, making it unsuitable for clinical development. Research has focused on phlorizin derivatives with a c-glucoside component, which provides increased resistance to enzymatic degradation and therefore increasing systemic bioavailability.
Dapagliflozin, canagliflozin, and empagliflozin are the most studied SGLT2 inhibitors and have been developed to satisfy the efficacy and safety criteria of the Food and Drug Administration (FDA) and European Medicines Agency (EMA). In addition, ipragliflozin, luseogliflozin, and tofogliflozin are available for clinical use in Japan, where the drug approval process places less emphasis on long-term safety. There are also several newer SGLT2 inhibitors at various stages of development. Ertugliflozin has entered Phase III development for the treatment of type 2 diabetes [5]. Sotagliflozin, a combined SGLT1 and SGLT2 inhibitor, has been studied in type 1 diabetes [6].
SGLT1 is the transporter responsible for glucose and galactose absorption in the gastrointestinal tract and early studies in type 1 diabetes suggest that this approach looks promising with pivotal trials planned [7]. Phase III studies in type 2 diabetes are pending.
2.2.2 Glycemic Effects of Dapagliflozin
The Phase III development program for dapagliflozin included 12 studies in 6998 patients with type 2 diabetes (Fig. 2.3). In a 24-week parallel group, double-blind, placebo-controlled trial in patients with a mean HbA1c of 7–10 % (n = 485), dapagliflozin significantly improved glycemic control with mean HbA1c changes of −0.23, −0.58, −0.77, and −0.89 % with placebo, 2.5, 5, and 10 mg dapagliflozin, respectively [8]. In another 24-week parallel group, double-blind, placebo-controlled trial with patients on metformin (n = 546), dapagliflozin significantly improved glycemic control with mean HbA1c changes of −0.3 % with placebo, −0.67 % with 5 mg, −0.7 % with 10 mg [9]. The glycemic control benefit was sustained in patients who completed the 102-week extension study (mean HbA1c changes of +0.02, −0.48, −0.58, and −0.78 % with placebo, 2.5, 5, and 10 mg dapagliflozin) [10].
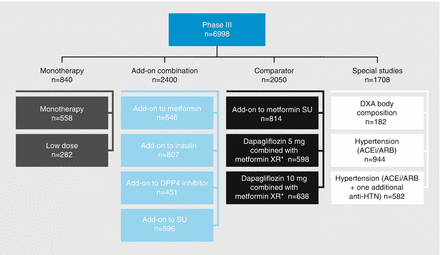

Figure 2.3
Dapagliflozin Phase III clinical development program. *Metformin XR is not approved or available in all countries. DPP-4 dipeptidyl peptidase-4, SU sulfonylurea
Similar improvements have been reported with dapagliflozin added on to glipizide (a sulfonylurea) and sitagliptin (a dipeptidyl peptidase-4 [DPP-4] inhibitor) [11, 12]. In a head-to-head randomized, 52-week, double-blind, active-controlled, noninferiority trial (n = 814) comparing dapagliflozin to glipizide, both drugs gave a significant reduction in HbA1c of 0.52 %, but did show significant differences in the secondary endpoints of weight loss and hypoglycemia [13]. Additionally, dapagliflozin has been studied as add-on to insulin in a 24-week, randomized, placebo-controlled trial followed by a 24-week extension period (n = 808) [14]. For the primary outcome (change in HbA1c from baseline to 24 weeks), mean HbA1c decreased from 0.79 to 0.96 % with dapagliflozin, compared to 0.39 % with placebo (mean difference, −0.4, −0.49, and −0.57 % in the 2.5, 5, and 10 mg dapagliflozin-dosing groups, respectively). Dapagliflozin has also been used in combination with modified-release metformin at 5 mg and 10 mg doses as initial treatment for type 2 diabetes when baseline HbA1c is high [15]. Two 24-week randomized trials (n = 598, n = 638) showed that the benefits of both agents were better than the individual components on HbA1c reduction (combined results showed reduction in HbA1c for dapagliflozin plus metformin XR −2.05 %, dapagliflozin alone −1.19 %, and metformin alone −1.35 %) [15]. The second study using 10 mg of dapagliflozin showed noninferiority to metformin.
2.2.3 Glycemic Effects of Canagliflozin
The Phase III development program for canagliflozin included ten studies in 7725 patients with diabetes (Fig. 2.4). In a 26-week, randomized, double-blind, placebo-controlled study (n = 587), canagliflozin at 100 mg or 300 mg significantly reduced HbA1c when compared with placebo (−0.77, −1.03, and 0.14 %, respectively) [16]. In a small sub-study (n = 127) of the CANagliflozin cardioVascular Assessment Study (CANVAS), patients were randomized to placebo, 100 mg canagliflozin, or 300 mg canagliflozin added to a sulfonylurea [17]. At 18 weeks, placebo subtracted changes were significant at −0.74 and −0.83 % for the 100 and 300 mg doses, respectively.
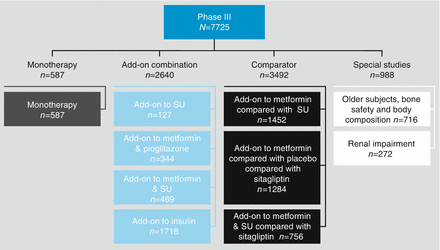

Figure 2.4
Canagliflozin Phase III clinical development program. SU sulfonylurea
In active comparator studies, canagliflozin has been added to metformin in dual therapy and compared to glimepiride and sitagliptin. In a 52-week, randomized, double-blind study (n = 1452), canagliflozin 100 mg was noninferior to glimepiride at lowering HbA1c (mean difference −0.01 %), and canagliflozin 300 mg was superior to glimepiride (mean difference −0.12 %) [18]. In a 26-week randomized, double-blind, four-arm, parallel group study in which patients on metformin were given placebo, sitagliptin 100 mg, or canagliflozin 100 mg or 300 mg, those given canagliflozin (100 and 300 mg) had reduced HbA1c vs. placebo (−0.79, −0.94, and −0.17 %, respectively) [19]. In a follow-up at week 52, patients given canagliflozin 100 and 300 mg demonstrated noninferiority, and canagliflozin 300 mg demonstrated superiority, to sitagliptin in lowering HbA1c (−0.73, −0.88, and −0.73 %, respectively) [19].
Two separate studies on the efficacy of canagliflozin as part of triple therapy (add-on to metformin/pioglitazone and add-on to metformin/sulfonylurea versus placebo) produced similar results, with both doses being superior to placebo, and the 300 mg dose more effective than 100 mg dose in lowering HbA1c [20, 21]. In a 52-week, randomized, double-blind, active-controlled study (n = 756) of subjects using stable metformin plus a sulfonylurea, canagliflozin 300 mg demonstrated noninferiority, and in a subsequent assessment superiority, to sitagliptin 100 mg (HbA1c reductions of −1.03 and −0.66 %, respectively) [22]. Canagliflozin has also been studied as add-on to insulin, with mean changes in HbA1c with canagliflozin 100 and 300 mg of −0.62 and −0.73 % at 18 weeks and −0.58 and −0.73 % at 52 weeks [23].
2.2.4 Glycemic Effects of Empagliflozin
The Phase III development program for empagliflozin included seven studies involving 5306 patients with diabetes (Fig. 2.5). In a 24-week randomized, placebo-controlled trial (n = 899) of empagliflozin with sitagliptin as an active comparator, the adjusted mean differences in change from baseline at week 24 were −0.74 % for empagliflozin 10 mg, −0.85 % for empagliflozin 25 mg, and −0.73 % for sitagliptin 100 mg [24]. In a 24-week, randomized, double-blind, placebo-controlled trial (n = 637) of empagliflozin as add-on treatment to metformin, changes from baseline in HbA1c were −0.13, −0.70, −0.77 %, with placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively [25].
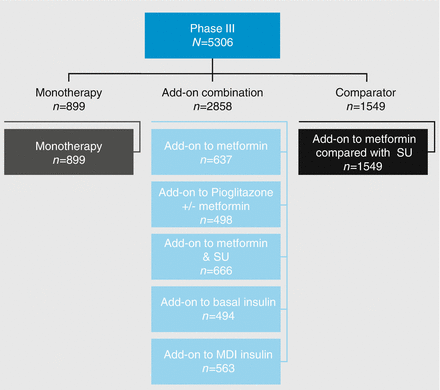

Figure 2.5
Empagliflozin Phase III clinical development program. MDI multiple daily injections, SU sulfonylurea
In a double-blind, placebo-controlled study with glimepiride as an active comparator (n = 1549) over 52 and 104 weeks, empagliflozin was found to be noninferior. At 104 weeks, the change from baseline in HbA1c with empagliflozin vs. glimepiride was −0.11 % [26]. In a 24-week, randomized, double-blind, placebo-controlled study (n = 666) of empagliflozin as add-on to metformin and gliclazide, the mean changes to baseline HbA1c were −0.17 % for placebo, −0.82 % for 10 mg empagliflozin, and −0.77 % for 25 mg empagliflozin [27].
Dual therapy with pioglitazone and the triple combination of empagliflozin, pioglitazone, and metformin have also been shown to be effective [28]. Empagliflozin has also been shown to be beneficial when added on to insulin. In a 78-week, randomized, double-blind, placebo-controlled trial adding empagliflozin to basal insulin gave mean HbA1c changes of 0, −0.6, and −0.7 % for placebo and empagliflozin 10 and 25 mg, respectively, with a reduction in insulin dose for those given empagliflozin [29]. Additionally, in a 52-week, randomized, double-blind, placebo-controlled study looking at empagliflozin as add-on to multiple daily injections of insulin, the mean HbA1c changes were −0.81, −1.18, and −1.27 % for placebo and empagliflozin 10 and 25 mg, respectively [30].
2.2.5 Glycemic Effects of SGLT2 Inhibitors: Where Are We Now?
The three Phase III development programs outlined for dapagliflozin, canagliflozin, and empagliflozin have trialed on a wide range of patients with diabetes. Efficacy has been demonstrated as a monotherapy and an add-on to the older oral therapies and insulin, when compared to placebo. Only dapagliflozin has been studied in the Phase III development program as add-on to a DPP-4 inhibitor and both dapagliflozin and canagliflozin have been studied with a DPP-4 inhibitor as a comparator. There are currently no completed Phase III studies looking at their use as add-on to glucagon-like peptide-1 (GLP-1) agonists or using these agents as comparators, which is likely to represent a large target population in real-life clinical practice. Only canagliflozin is currently in the recruitment stage of a Phase III study as add-on to a GLP-1 agonist [31].
Additional uncertainty remains as to whether one SGLT2 inhibitor is better than the others at lowering blood glucose, as there have been no head-to-head Phase III clinical studies with this as the primary outcome. However, there is one small study (n = 54) looking at the pharmacodynamic effects of 10 mg of dapagliflozin vs. 300 mg of canagliflozin that suggested the latter had a slightly more potent effect in healthy volunteers, as measured by postprandial plasma glucose excursions, 24-h urinary glucose excretion, and renal threshold for glucose excretion [32]; how this translates into clinical practice and the management of diabetes remains unclear.
2.3 Effects of SGLT2 Inhibitors on Body Weight
Obesity and particularly visceral/abdominal obesity is associated with diabetes, insulin resistance, metabolic syndrome, and increased cardiovascular risk. Weight gain is a side effect of insulin therapy, sulfonylureas, and thiazolidinediones, whereas metformin and DPP-4 inhibitors are weight neutral. GLP-1 agonists such as exenatide and liraglutide are associated with weight loss. Weight reduction has been a positive secondary outcome that has been consistently demonstrated in the Phase III studies of all available SGLT2 inhibitors. Mean weight changes at 24 weeks for placebo vs. dapagliflozin as initial therapy were −2.2 and −3.3 kg, respectively [8]. When added to insulin, the mean weight changes at 24 weeks for placebo vs. dapagliflozin were +0.43 and −2.04 kg (these results reflect lower insulin requirements for the active treatment group). In a 102-week study of dapagliflozin added to metformin, all doses reported sustained weight loss (−1.10 to −1.74 kg, as compared to weight gain with placebo) [10]. Similar findings have been reported for canagliflozin and empagliflozin, with weight loss in short-term studies when using them as first-line, as well as weight loss when added to drugs that are known to cause weight gain, including insulin. Weight loss benefits appear to be sustained [10].
Initial weight loss for SGLT2 inhibitors may be due to the osmotic diuretic effect of treatment. However, sustained weight loss over the subsequent weeks is a consequence of caloric loss due to glycosuria. The glucose excreted in the urine equates to net loss of 200–300 cal per day. As well as reduction in total body weight, dapagliflozin has been shown to significantly reduce waist circumference (−1.52 cm) when compared to placebo [33]. In this study, approximately two-thirds of the weight loss was attributable to reductions in fat mass. The reductions in total body weight, fat mass, and waist circumference occurred in the context of sustained and significant glycosuria, which supports the theory that this was due to caloric loss. Whole body dual-energy X-ray absorptiometry demonstrated the reduction in total body weight was due to loss in fat mass, and not loss of fluid or lean mass. It also demonstrated an initial rapid decline in weight over the first week, followed by a more gradual decline that had not plateaued at 24 weeks. This, coupled with a partial rebound in weight after discontinuation, suggests that diuresis may contribute to the initial weight loss, and loss in total body fat is predominant after this. However, in a study of 86 patients with type 2 diabetes, there was evidence that weight loss did plateau as a result of increased calorie intake, as measured urinary glucose excretion did not lead to the weight loss expected [34].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree