Unusual Sites of Arterial Occlusion
Philip A. Kalra
Jon G. Moss
Peter Wiedemann
UNUSUAL SITES OF ARTERIAL OCCLUSION
In this chapter, we consider occlusive arterial diseases as they affect three separate vascular sites, the renal, gut, and retinal vessels. In the renal vasculature, most efforts have been directed at diagnosing and treating significant renal artery narrowing, as opposed to complete occlusion, whereas the latter is more relevant in the clinical presentation of intestinal or retinal vascular diseases.
RENAL ARTERY STENOSIS AND OCCLUSION
Introduction
Renal artery narrowing, which is usually focal but can be both multiple and more diffuse, is termed renal artery stenosis (RAS), and it is of clinical importance because of its blood flow-limiting effects upon the renal circulation and consequent intrarenal ischemia. This in turn can perturb renal function, blood pressure control, and renal neuroendocrine activity, with clinical manifestations including fluid and salt retention. Interest in RAS accelerated during the 1980s and 1990s with the advent of interventional procedures that could dilate stenotic lesions, providing the possibility that renal revascularization might improve patient outcomes. However, recent trials have shown that such intervention for atherosclerotic lesions, by far the commonest cause of RAS, may not be associated with patient benefit in the majority and that there are still gaps in our knowledge regarding management of the condition.
Etiology
Atheromatous change within the main renal arteries is very commonly seen in ageing populations, but also in younger patients with risk factors or a predisposition for early development of atheroma, and hence atherosclerosis is the cause of over 90% of RAS in Western populations; the remainder is due to fibromuscular disease (FMD). The epidemiology is different in the Indian subcontinent and the Far East, with vasculitis, including Takayasu arteritis, said to be responsible for up to 60% of RAS cases.1 The latter is discussed in Chapter 100.
As the epidemiology, clinical presentations, and outcomes after treatment of FMD and atherosclerotic RAS differ markedly, they are considered separately, with greatest attention being directed to atherosclerotic renovascular disease (ARVD), which is the preeminent condition.
Fibromuscular Disease
Epidemiology and Pathology
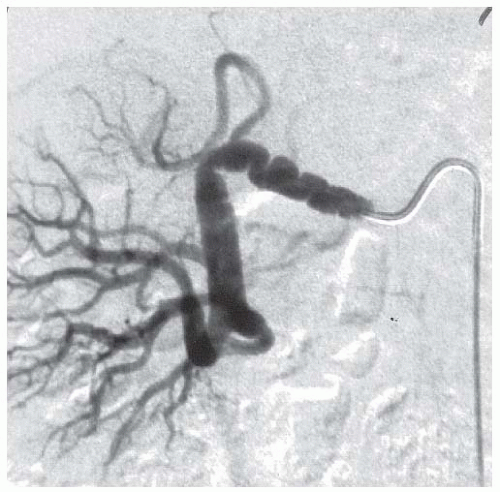
FMD accounts for 5% to 10% of RAS cases. However, autopsy studies and analysis of renal vascular anatomy in healthy renal donors suggest that its prevalence maybe as high as 2% in Western general populations.2 FMD involves the renal arteries in 60% to 75% of cases, but it can involve other major arteries such as the carotids (in 30% to 60%), vertebral, mesenteric, celiac axis, and coronary vessels.3 The pathogenesis of FMD is unknown, but there is suspicion that female hormones could be important, given the fact that up to nine times more females than males are diagnosed with the condition. Several different pathologic forms are recognized, the most common of which is medial fibroplasia, typically found in women who present in their third or fourth decades. Here the noninflammatory pathology involves areas of thinning of the intima and media of the vessel wall with loss of the elastic lamina, changes that lead to aneurysm formation. These areas alternate with localized regions of fibrotic narrowing, and so the result is the classical “string of beads” appearance at angiography (FIGURE 95.1). Stenoses tend to occur more distally in the renal circulation than atherosclerotic lesions, and they can be several centimeters in length and also present in multiple vessels; bilateral renal FMD is seen in 30% to 50% renal cases.
Clinical Picture
The classical presentation of FMD is with hypertension and usually well-preserved renal function in young adults, although there is a growing consensus that many cases present in later life. The diagnosis should certainly be considered in young patients (e.g., <35 years old) who present with severe or accelerated-phase hypertension, or in other patients where there is new-onset hypertension, deterioration in previously stable hypertension, or renal functional deterioration in patients treated with renin angiotensin blocking (RAB) agents. An abdominal bruit may be detected, but awareness of the possibility that neurologic features, mesenteric angina, or claudication may be manifestations of FMD at nonrenal arterial sites is important. Progressive narrowing of the renal arteries may be seen in a third of patients over time, but progression to complete occlusion is rare.
Management
The management of patients with fibromuscular RAS is centered upon control of hypertension. Medical therapy, most appropriately utilizing RAB agents, may be suitable in the majority. Renal revascularization with percutaneous renal angioplasty (PTRA)
is indicated for patients in whom blood pressure cannot be controlled to target via medical therapy, in those with accelerated hypertension, and in those intolerant of RAB. Unlike the situation for atherosclerotic RAS, success rates for hypertension control after PTRA are good in FMD, with cure (i.e., no further need for antihypertensive drugs) observed in 30% to 60% and an improvement in blood pressure control in the majority of the remaining cases.4 The 10% of patients unresponsive to PTRA are more likely to include those with multiple FMD lesions or with intrarenal disease.
is indicated for patients in whom blood pressure cannot be controlled to target via medical therapy, in those with accelerated hypertension, and in those intolerant of RAB. Unlike the situation for atherosclerotic RAS, success rates for hypertension control after PTRA are good in FMD, with cure (i.e., no further need for antihypertensive drugs) observed in 30% to 60% and an improvement in blood pressure control in the majority of the remaining cases.4 The 10% of patients unresponsive to PTRA are more likely to include those with multiple FMD lesions or with intrarenal disease.
Atherosclerotic Renovascular Disease
Atherosclerotic narrowing of the renal arteries is by far the commonest cause of RAS.
The renovascular disease usually develops as part of a systemic inflammatory atheromatous syndrome and is accompanied by macrovascular disease in several other important vascular beds, notably the coronary, aortoiliac, and iliofemoral circulations. The term ARVD is preferentially used to describe all patients with atherosclerotic renal disease as around 40% have evidence of renal artery occlusion (RAO) at first clinical presentation. About 90% of RAS lesions are “ostial,” that is occurring within 1 cm of the origin of the renal artery, and around 35% of patients have bilateral disease.5
Epidemiology and Risk Factors
ARVD is a disease of ageing. In our own local renovascular database, which comprises almost 1,000 patients with ARVD referred from a 1.5 million population over 20 years, the median age of the population is 70 years with 46% of patients in their seventh decade of life and 87.1% of patients being aged >60 years at study entry.6 As would be expected, prevalence is increased in smokers and in patients with type 2 diabetes, and epidemiologic studies demonstrate that about 30% of typical ARVD cohorts are diabetic.7 Although there is no clear association of ARVD with hyperlipidemia, the lipoprotein profile is similar to that found in other vascular diseases, with a very low apolipoprotein A1 concentration but only slight elevations of total and LDL cholesterol.8
The epidemiology of ARVD differs according to the studied population. Many patients have asymptomatic ARVD that is only identified during the investigation of other arterial conditions, and so an accurate prevalence figure for the general population is unknown. However, in North Carolina, 6.8% of 834 community-based people aged >65 years were shown to have incidental RAS of >60% by Doppler imaging.9 Approximately, 1 in 200 (0.54%) US citizens aged over 65 years and receiving Medicare had a diagnosis of ARVD in 1998 to 1999,7 but the prevalence is far greater in groups enriched with vascular disease, such as those with coronary artery disease (CAD) or peripheral vascular disease (PVD). Medicare data derive from the 5% annual random sample of patients aged >65 years available for analysis. New cases of ARVD were diagnosed with an incidence of 3.7 per 1,000 patient years (0.37% per year) in 2000 to 2001.7 A study of 12 years of Medicare data (1992 to 2004) has shown a 3.35 times increase in ARVD diagnosis over the period, presumably reflecting a change in investigational and screening strategies rather than any change in frequency of disease development.10
Association of ARVD with Other Vascular Diseases
ARVD is often identified as an incidental or asymptomatic pathology in patients with other vascular diseases, and so it is of particular interest to the disciplines of cardiology, vascular surgery, and hypertension. Many studies have been undertaken in selected groups of patients who are likely to be enriched with ARVD. The most extensive interest in the association of ARVD with CAD has been developed by the group in Duke University Medical Centre in patients presenting predominantly with angina or myocardial infarction.11 Up to 11% of these patients had significant unilateral RAS and 4% bilateral RAS >50% when investigated with abdominal aortography concomitant with coronary angiography. Patients with greater extent of CAD have greatest severity of RAS. As will be discussed later, the presence of RAS in this population indicates an adverse prognosis, which in part resulted in cardiologic enthusiasm to detect (“shoot the renals”) and perform endovascular intervention (“drive by angioplasty”) of RAS lesions during coronary investigation. In view of the frequent association of ARVD with CAD and hypertension, it is little surprise that structural heart disease can be detected in many patients with RAS and that syndromes of cardiac dysfunction are highly prevalent. Interest in the relationship between congestive heart failure (CHF) and RAS has been increasing, and estimates suggest that 30% to 50% of older CHF patients may have ARVD.12,13 Clinical presentations with sudden-onset or “flash” pulmonary edema can be life-threatening and may be the first indication of ARVD in up to 10% of patients.14 Its true incidence is most probably underestimated due to the fact that renovascular investigation is undertaken in a minority of patients with abrupt-onset left-ventricular failure. Patients with bilateral significant RAS or a solitary functioning kidney are those at most risk of this condition, which is one of the accepted indications for renal revascularization. As would be anticipated due to anatomical relationships, aortoiliac disease is
frequently associated with ARVD, with RAS often being detected incidentally during vascular surgical investigation. The prevalence of ARVD in patients undergoing aortography for intermittent claudication has been found to be 30% to 45%, and the presence of ≥60% RAS in patients with PVD has been associated with a 2.5-fold increased risk for major cardiovascular events and death during follow-up.15 Carotid disease also frequently coexists with ARVD; in a Japanese study involving 729 high-risk cardiovascular patients, 22% of those with carotid artery stenosis had RAS. Conversely, carotid disease has been demonstrated in 46% of patients with RAS.16 The above macrovascular disease prevalence figures are further emphasized in studies of the US Medicare population, and a study of ARVD patients in 20 hospitals in Spain found PVD present in 67.5%, CAD 44.8%, heart failure 32.6%, and stroke in 27.3%.17
frequently associated with ARVD, with RAS often being detected incidentally during vascular surgical investigation. The prevalence of ARVD in patients undergoing aortography for intermittent claudication has been found to be 30% to 45%, and the presence of ≥60% RAS in patients with PVD has been associated with a 2.5-fold increased risk for major cardiovascular events and death during follow-up.15 Carotid disease also frequently coexists with ARVD; in a Japanese study involving 729 high-risk cardiovascular patients, 22% of those with carotid artery stenosis had RAS. Conversely, carotid disease has been demonstrated in 46% of patients with RAS.16 The above macrovascular disease prevalence figures are further emphasized in studies of the US Medicare population, and a study of ARVD patients in 20 hospitals in Spain found PVD present in 67.5%, CAD 44.8%, heart failure 32.6%, and stroke in 27.3%.17
ARVD Prevalence in Other Populations
In selected hypertensive populations, up to 2% of patients can be shown to have RAS, but it is quite likely that the lesion is not causal in most and that “renovascular hypertension” exists only in the minority. This assertion is based upon the fact that improvements in blood pressure are the exception rather than the rule after revascularization in the majority of patients with RAS, whereas a rigorous definition of renovascular hypertension would be cure or substantial improvement in hypertension after such procedures. Similarly, although impaired renal function is found in most patients with ARVD, its etiology is usually related to other factors causing renal injury, such as hypertension and an atherogenic milieu that predate RAS development, and the hemodynamic effects of a RAS may not necessarily be pathogenetically important. Up to 15% of patients with end-stage kidney disease (ESKD) on dialysis programs have ARVD, but in many cases the disease is a comorbid association rather than a cause of the ESKD.18 Despite this, awareness of the association is important as some cases of ARVD will present subacutely with ESKD, and revascularization of critical RAS can occasionally lead to independence from a future of dialysis. Patients with ESKD have the poorest prognosis of all ARVD patients, presumably because of their extensive vascular comorbidities, and this will be discussed later.
Clinical Presentations of ARVD
A large proportion of patients diagnosed with ARVD are asymptomatic from the condition, and their disease is identified during investigation of other diseases (e.g., during coronary or peripheral angiography). Others are identified after referral to specific clinics (e.g., nephrology, hypertension, or heart failure clinics), whereas a minority are diagnosed during an acute hospital admission (e.g., acute kidney injury [AKI], pulmonary edema). Hypertension is usually present in patients with anatomically significant RAS; data from the angioplasty and stenting for renal artery lesions (ASTRAL) trial show that 98% of ARVD patients had this diagnosis at enrolment.19 The typical pattern is of systolic hypertension with low diastolic pressure and widened pulse pressure, often resistant to medical therapy. Perhaps 2% of ARVD presentations are with AKI, the most likely scenarios being bilateral critical RAS (e.g., >95% RAS), acute RAO, in association with accelerated-phase hypertension or with cholesterol atheroembolization in patients with severe aortic atheroma, radiocontrast injury as a result of angiographic procedures or, most commonly, following the use of RAB therapy.
The most frequent nephrologic presentation is with asymptomatic chronic kidney disease (CKD), usually associated with hypertension, in patients referred to the nephrology clinic. The link between ARVD and sudden-onset heart failure, or CHF, has already been described, but many additional patients with primarily diastolic heart failure and hypertension have underlying contributory RAS. With all the above clinical presentations, a key pointer to the diagnosis of RAS is the presence of arterial bruits (epigastric and femoral).
Diagnosis of ARVD
ARVD should be suspected in patients with hypertension and/or CKD and any of the following:
Audible vascular bruits
>1.5 cm disparity in bipolar renal length on renal imaging
Unexplained pulmonary edema
Unexplained CKD in those with evidence of other vascular disease
Significant deterioration of renal function in association with RAB therapy
There are several imaging techniques that can be used to diagnose RAS (FIGURE 95.2). Magnetic resonance angiography (MRA) has a sensitivity/specificity for detection of RAS exceeding 90%, but it can overestimate the degree of RAS. Contrast MRA should be avoided where possible in patients with stage 4 or worse CKD (glomerular filtration rate [GFR] of <30 mL/min) because of the risk of nephrogenic systemic fibrosis, a syndrome that can
be induced by certain preparations of gadolinium.20 Computed tomography angiography (CTA) or multislice CT has similar sensitivity/specificity to MRA for RAS detection and evaluation (FIGURES 95.3 and 95.4). Its main limitation is risk of contrast nephropathy in patients with advanced CKD.21 The likelihood of this complication occurring can be minimized by prophylactic protocols involving intravenous fluid infusion. Duplex ultrasonography is highly sensitive for the detection of RAS, and the Doppler waveforms provide information on intrarenal vascular resistance, which provides some indication of the extent of intrarenal parenchymal disease.22 Limitations of the technique are patient body habitus and its operator dependency, but it is completely risk free and is the best screening tool for RAS in patients with advanced CKD. Conventional renal angiography (usually intra-arterial angiography) is utilized only to confirm the presence of RAS at the time of revascularization procedures, or in diagnosis of more complicated or uncertain cases. It only provides two-dimensional images, it is invasive, and use of contrast carries a risk of contrast nephropathy.
be induced by certain preparations of gadolinium.20 Computed tomography angiography (CTA) or multislice CT has similar sensitivity/specificity to MRA for RAS detection and evaluation (FIGURES 95.3 and 95.4). Its main limitation is risk of contrast nephropathy in patients with advanced CKD.21 The likelihood of this complication occurring can be minimized by prophylactic protocols involving intravenous fluid infusion. Duplex ultrasonography is highly sensitive for the detection of RAS, and the Doppler waveforms provide information on intrarenal vascular resistance, which provides some indication of the extent of intrarenal parenchymal disease.22 Limitations of the technique are patient body habitus and its operator dependency, but it is completely risk free and is the best screening tool for RAS in patients with advanced CKD. Conventional renal angiography (usually intra-arterial angiography) is utilized only to confirm the presence of RAS at the time of revascularization procedures, or in diagnosis of more complicated or uncertain cases. It only provides two-dimensional images, it is invasive, and use of contrast carries a risk of contrast nephropathy.
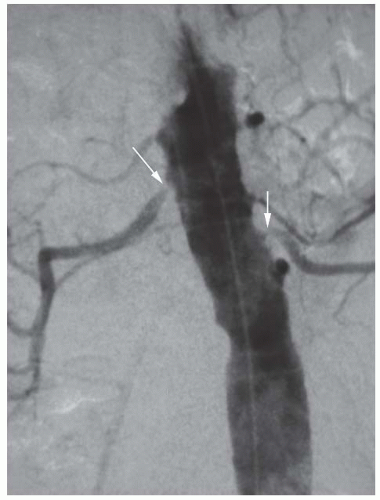 FIGURE 95.2 Intra-arterial digital subtraction angiogram showing severe bilateral atherosclerotic renal artery stenosis (arrowheads). |
Treatment of ARVD
Medical Therapy
ARVD occurs as part of a diffuse inflammatory vascular disease process, and extrarenal vascular pathology (e.g., CAD, heart failure, cerebrovascular accident) is a major determinant of the poor outcome of the patients. Hence, although there is no evidence base to guide optimal vascular protective therapy, a sensible approach is to advise smoking cessation, and treatment with antiplatelet therapy and statins. The latter can stabilize atherosclerotic plaques, and there is also some evidence that they can ameliorate progression or even induce regression of atherosclerotic RAS.23 The target blood pressure of <130/80 should be sought, but this may only be attained in a minority of patients, even when multiple antihypertensive agents are used in combination. Use of RAB therapy, although seemingly counterintuitive, is actually the most sensible approach for the majority of ARVD patients, especially for those with coexisting CAD and cardiac dysfunction, or proteinuria (indicative of chronic renal parenchymal injury). Careful monitoring of renal function is obviously required, particularly in patients with significant bilateral RAS, or RAS affecting a solitary kidney.
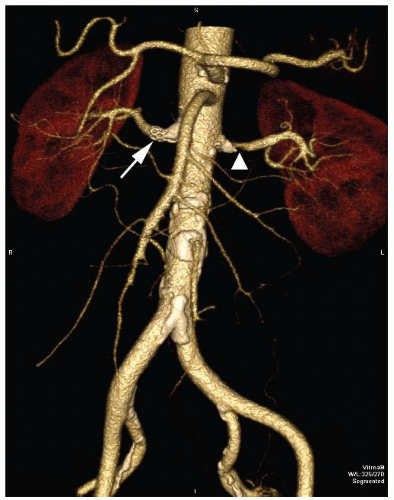 FIGURE 95.4 Spiral CT scan showing the quality of three-dimensional postprocess imaging. There is a left RAS (arrowhead) and a right renal artery stent (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|