Chapter 44 Uncommon Thoracic Tumors
Thymic Tumors
The thymus gland is a bilobed lymphoepithelial organ located in the anterior mediastinum, behind the sternum and in front of the great vessels. In early life, the thymus functions in T-lymphocyte differentiation and maturation and releases T lymphocytes into the circulation. It weighs 12 to 15 g at birth and 40 g at puberty, and during adulthood, it slowly involutes and is largely replaced by adipose tissue. The thymus is composed of an outer cortex consisting primarily of epithelial cells, degenerated keratinized epithelial cells (Hassall’s corpuscles), myoid cells, thymic lymphocytes (“thymocytes”), and B lymphocytes, which form rare germinal centers.12
Although various tumors and cysts can arise in the thymus, tumors of the thymus are uncommon. Most thymic tumors arise in the epithelial cells of the thymus. They account for approximately 50% of anterior mediastinal masses.13,14 The most common thymic tumors are thymoma, thymic carcinoma, and thymic carcinoids. Thymomas, 90% of which are found in the anterosuperior mediastinum, are commonly associated with myriad paraneoplastic syndromes. They are the most common tumor found in the anterior mediastinum.13,15 Thymomas are associated with an exuberant lymphoid component composed of immature cortical thymocytes. Although they appear benign histologically, they may exhibit invasive behavior clinically.
Thymic carcinomas (i.e., type C thymomas) also arise in the thymic epithelium, but they have a higher propensity for capsular invasion and widespread metastases, especially those with features of high-grade malignancy. The histologic subtypes include clear cell carcinoma, sarcomatoid carcinoma, and anaplastic carcinoma. Low-grade thymic carcinomas (well-differentiated squamous cell carcinomas, basaloid carcinomas, and mucoepidermoid carcinomas) also exist and are characterized by a relatively more favorable clinical course, with a lower incidence of local recurrence and metastasis.16,17
Neuroendocrine tumors of the thymus are rare, accounting for less than 5% of all neoplasms of the anterior mediastinum. Like carcinoids at other sites, they are thought to originate from endodermal cell (or foregut cellular) precursors. Unlike carcinoids in other locations, most thymic carcinoids behave aggressively. They often invade locally and commonly metastasize to regional lymph nodes.18 Approximately 50% of patients may develop endocrine abnormalities.19
Etiology and Epidemiology
The etiology of thymic tumors is largely unknown. There is a reported association between Epstein-Barr virus (EBV) infection and tumors of the thymus. For instance, thymic diseases are more common in Far Eastern countries, where the incidence of EBV infection is high.20,21 Defective viral genomes have been isolated in patients with lymphoepithelioma-like thymic carcinoma.21–24 Childhood thymus irradiation has been linked to the development of thymic tumors,25 and familial cases have been reported, suggesting a possible relationship with cytogenetic abnormalities.26,27 In an analysis of secondary or concomitant neoplasms in 1495 patients with acute lymphoblastic leukemia enrolled in two consecutive multicenter protocols, an increased risk of solid tumors was identified.28 Although the histologic characteristics of these second malignant tumors varied, thymoma was among the solid tumors identified in an adult survivor of acute lymphoblastic leukemia. Among patients with primary thymic carcinoid tumors, up to 30% were reported in the setting of multiple endocrine neoplasia type 1 and 2.29,30 Other studies have reported a distinctive chromosome abnormality involving translocation of fragments of chromosomes 15 and 19 [t(15:19)(q15:p13)] in thymic carcinoma, particularly in young adults and pediatric patients with high-grade thymic carcinoma.31–33 Deletion of the short arm of chromosome 6 is associated with benign thymomas.34 However, the molecular mechanisms of thymic tumor oncogenesis are largely unknown. Aberrations involving chromosome 6 suggest that several putative tumor suppressor genes may contribute to the pathogenesis of thymoma.35
In a small study of 37 cases, most type A thymomas did not show any chromosomal aberration, whereas type B3 thymomas and thymic carcinoma (i.e., type C thymoma) shared some genetic aberrations, including the loss of chromosome 6 and the gain of chromosome 1q.36 The loss of chromosome 6, on which the human leukocyte antigen locus and some of the tumor suppressor genes have been identified, and the gain of chromosome 1q, to which growth promoter genes have been mapped,36–38 may play a role in tumorigenesis and pathogenesis of the paraneoplastic autoimmunity characteristics of thymoma. Such chromosomal aberrations have been implicated in a variety of human neoplasms.39,40
The true incidence of thymoma is unknown, and the annual incidence in the United States has not been well defined. The SEER program reported a thymoma incidence of 0.13 to 0.15 per 100,000 people.1 Although thymomas are uncommon, they are the most frequently diagnosed mediastinal masses, representing approximately 30% of anterior mediastinal lesions and 20% of all mediastinal tumors in adults.13–15,42–45 Most patients with thymoma are between the ages of 40 and 60 years, with a median age of 52 years and an equal overall gender ratio.46–49 Thymomas are less common in children, accounting for approximately 15% of anterior mediastinal masses.15,46–49
Thymic carcinomas are distinct from invasive thymomas both pathologically and clinically and carry a poorer prognosis.50 They account for 5% to 36% of all thymic neoplasms.51,52–54 The wide range in incidences from various studies reflects differences and changes in the pathologic classification of this rare tumor. Patients with thymic carcinoma are typically middle-aged or elderly, and there is a slight male predominance. Thymic carcinoids represent less than 5% of anterior mediastinum lesions55,56 and typically affect middle-aged men.16
Prevention and Early Detection
A few small subgroups of cases are associated with thymic irradiation during childhood, EBV infection (associated with lymphoepithelioid thymic carcinoma), or familial cytogenetic abnormalities; the etiologic factors for most thymic tumors are unknown, however, and there is no known mechanism for prevention. Thymomas are characterized by an indolent growth pattern with a tendency toward local invasion. Thymomas are associated with autoimmune disorders, particularly myasthenia gravis, in 30% to 45% of cases.57,58 Most thymic tumors are discovered incidentally because of abnormalities observed on routine radiographs or during evaluation for myasthenia gravis. Approximately 10% to 15% of patients with myasthenia gravis have benign or malignant thymomas.59,60,61–63 Thymic carcinomas and carcinoids are commonly detected incidentally. They are locally invasive and are found to have metastasized to regional lymph nodes and distant sites in up to 30% of patients at diagnosis.18,52,64
Biologic Characteristics and Molecular Biology
Thymomas are usually slow-growing tumors with an indolent natural history. Although histologically benign, they spread by direct extension and are capable of local and regional invasion and have a marked tendency for recurrence in the mediastinum and pleura. Intrathoracic recurrence can develop after complete surgical resection in up to 40% of those with invasive disease.65 Late relapse is not uncommon.60,66,67 Metastases are typically confined to the pleura, pericardium, or diaphragm.68 Nodal and hematogenous metastases may occur but are rare.45,69 An increased risk for second malignant tumors has been reported.70,71,72
Patients with thymoma may have dysregulation of the lymphocyte selection process associated with abnormal proliferation, autoimmunity, and immunodeficiency. Thymoma-associated autoimmune disease involves an alteration in circulating T-cell subsets.73–75 Up to 70% of thymomas may be associated with paraneoplastic syndromes.51 Various autoantibodies have been described.76,77 In addition to T-cell defects, B-cell lymphopenia has been observed in thymoma-related immunodeficiency along with hypogammaglobulinemia (i.e., Good’s syndrome).78,79
Factors influencing the different biologic behaviors of thymoma subtypes are poorly understood. Some pathologic features, such as altered expression of TP53 and BCL2,80,81 counts of argyrophilic nucleolar organizer regions,82,83 proliferating cell nuclear antigen, matrix metalloproteinases, and Ki-67 index,82,84 are associated with biologic behavior, clinical stage, and prognosis.85 There is a tendency for up-regulation of adhesion and co-stimulatory molecules in thymoma.86–89 Altered TP53 expression may be implicated in the initial stages of tumorigenesis, and increased expression of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR) may play a role in thymoma genesis.90 A decreased OS time is predicted by Src kinase and TP53 co-expression.91 Recently, it has been proposed that a deficiency in the autoimmune regulator (AIRE) gene has an oncogenic effect on thymomas. AIRE expression is lacking in approximately 95% of thymomas; therefore loss of AIRE expression may provide a potential mechanistic link for the well-recognized association of autoimmunity with thymoma.92,93
Thymic carcinomas possess overt features of malignancy similar to those of carcinoma arising in any other organ, with a higher propensity for capsular invasion and metastases than invasive thymomas. Associated paraneoplastic syndromes occasionally exist with well-differentiated lesions.94,95 Most variants of thymic carcinoma are highly lethal, producing frequent metastases to regional lymph nodes, bone, liver, and lung.96–98 Thymic carcinomas may express high levels of EGFR,90,99,100 but EGFR gene mutations are rare.101 Tumor and serum levels of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) can be elevated,102,103 and correlations among such growth factors, angiogenesis, and invasiveness have been observed.102,104 Thymic carcinoma has been associated with increased expression of epithelial membrane antigen, cytokeratin subtypes, and TP53 protein.80,105–109 Somatic KIT gene mutations are present in some thymic carcinomas but not in thymomas.110 Increased expression of CD70 may serve as a marker for thymic carcinoma.111 Other surface antigenic molecules, such as CD5 and CD99, have aberrant expression in thymic carcinoma.112–114 Cytoplasmic phosphorylated AKT serine 473 and insulin-like growth factor-1 receptor (IGF-1R) are expressed in a higher proportion of recurrent tumors and more aggressive subtypes according to the World Health Organization (WHO) staging system. IGF-1R was associated with a worse progression-free survival time.115 Thymic carcinomas can be distinguished from lung carcinomas by negative expression of thyroid transcription factor-1105 and a distinctly different cytokeratin profile.116 Additionally, although mutations in the TP53 gene are common in lung carcinoma, TP53 gene mutation was not found in any of seven examined thymic carcinomas.110
Thymic carcinoids have a similar malignant clinical course with a poor prognosis, frequently worse than carcinoids of the gastrointestinal tract. They may secrete peptides, amines, kinins, prostaglandins, or other substances. In a study of 80 patients with thymic carcinoids, 74% were male, 72% had related symptoms, 6% had Cushing’s syndrome, and 16% had other endocrine abnormalities.117 Even after complete resection, recurrence is common (range, 36% to 87% of patients).18,117,118 OS rates were only 28% and 10% at 5 and 10 years, respectively.
Pathology and Pathways of Spread
There is no direct correlation between the histopathology of thymomas and their malignant potential.49,119,120 However, an accurate histologic diagnosis is still crucial in patient management.
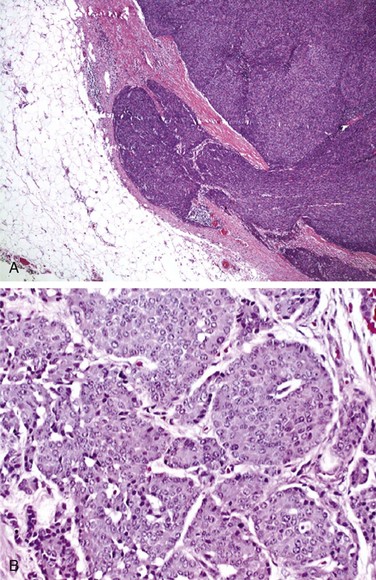
Grossly, thymomas are nodular, multilobulated, and firm. They may contain cystic spaces, calcification, or hemorrhage, and they may be neatly encapsulated, adherent to surrounding structures, or invasive. Thymomas generally display two cell types: epithelial and lymphatic cells. They are classified as predominantly epithelial, predominantly lymphocytic, mixed lymphoepithelial, or spindle cell type. Morphologically, these cells are rather large and may be round, oval, or spindle shaped with vesicular nuclei and small nucleoli. The cytoplasm is often eosinophilic or amphophilic. Thymic neoplasms arise from epithelial cells (Fig. 44-1). The lymphocytic component is mostly made up of normal-appearing mature lymphocytes. Some of the other microscopic features that may be seen in thymomas include Hassall’s corpuscles, keratinizing squamous epithelium, rosettes, glands, cysts, papillary structures, and germinal centers. Immunohistochemistry testing is often helpful in making the diagnosis. Thymomas typically stain positive for a number of thymic epithelial markers, including cytokeratin, thymosin ß3 and α1, and epithelial membrane antigen.
Thymic carcinoma histologic characteristics are more diverse. The tumor is cytologically malignant, and the largest proportion of cases have squamous cell histologic findings. Other subtypes include lymphoepithelioma-like carcinomas, clear cell carcinomas, sarcomatoid carcinomas, adenosquamous carcinomas, mucoepidermoid carcinomas, adenocarcinomas, and basaloid squamous cell carcinomas.16 Their histologic appearances are not different from carcinomas in other sites.121
The histologic features of thymic carcinoids are identical to carcinoid tumors in other organs. Unlike thymomas, they are rarely encapsulated. Immunohistochemically, they may stain positively with CAM 5.2, low-molecular-weight cytokeratins, chromogranins, synaptophysin, and leucine-7.117
The predominant pattern of spread of thymomas is by direct invasion into adjacent organs. The degree of encapsulation and the invasion of adjacent tissues define malignancy for these tumors rather than the histologic appearance.66 Approximately 50% of cases in surgical series are noninvasive.* Thymomas may metastasize as implants on pleural surfaces or pulmonary nodules, but they rarely metastasize to extrathoracic areas.45 When thymomas metastasize, the most common site is the pleural cavity, in which they form pleural plaques, malignant pleural effusions, and diaphragmatic masses. Invasion into the superior vena cava, brachiocephalic vein, lung, and pericardium may be observed.127
Thymic carcinomas invade locally, often involve the pleura and mediastinal nodes, and sometimes spread regionally to the cervical and axillary lymph nodes.128 Distant metastases to the lungs, liver, brain, and bone also occur. Distant metastases to bone, liver, or skin occur in 30% to 40% of cases of thymic carcinoids,19 and they may be seen in 70% of patients within 8 years from initial diagnosis.129
Clinical Manifestations and Patient Evaluation
Approximately 30% to 40% of patients with thymomas, thymic carcinomas, or carcinoids are asymptomatic and are discovered incidentally.45,48,58,130 Clinical symptoms vary greatly, depending on the size of the tumor and effect on adjacent structures, but they are usually those of a mediastinal mass producing cough, chest pain, dyspnea, hoarseness, superior vena cava syndrome, and symptoms related to tumor hemorrhage.16,131,132 Patients may also have dysphagia, fever, weight loss, and anorexia.
Some thymomas present with symptomatic paraneoplastic syndromes; the most common is myasthenia gravis, which is seen in approximately 45% of patients.58 Box 44-1 lists other syndromes.* Thymic carcinoids may also be associated with Cushing’s syndrome, Eaton-Lambert syndrome, syndrome of inappropriate secretion of antidiuretic hormone (SIADH), and hypercalcemia,19 but the classic carcinoid syndrome is rare. The causes of these syndromes remain obscure, although autoantibodies have been demonstrated, mostly in thymoma patients.76,77,137
Myasthenia gravis is an autoimmune neuromuscular junction disorder characterized by the presence of antiacetylcholine receptor antibodies, which cause an acetylcholine receptor deficiency at the motor end plate. The disease is characterized by rapid exhaustion of voluntary muscular contractions, with a slow return to the normal state.138,139 Myasthenia gravis is common with thymoma but rare in thymic carcinoma. Patients with thymoma and myasthenia gravis may have an increased operative mortality rate, and most surgical deaths are attributed to myasthenic crisis. Death of patients with thymoma and myasthenia gravis is commonly caused by complications of myasthenia gravis, whereas in patients without myasthenia gravis, death often is attributed to local progression of tumor.124 The overall long-term prognosis does not appear to be adversely affected by the presence of myasthenia gravis.60,124
Prognostic Factors
The two most important prognostic factors for thymoma are invasiveness (stage) and completeness of surgical resection.† Capsular invasion is commonly used as the basis for designation as benign or malignant. Tumor size (>10 cm) and the presence of symptoms also have prognostic value.43,143,144 Patients with complete or radical excision have significantly improved survival times over those with subtotal resection or biopsy only.141,145 Although almost all noninvasive thymomas can be totally resected, the ability to achieve a complete resection in invasive cases varies from 58% to 73%.60,125,141 Older series reported a poor prognosis associated with myasthenia gravis,46,47,126 but several modern series have failed to confirm this observation.* Myasthenia gravis may even confer a survival advantage because neuromuscular symptoms may lead to earlier discovery of a small thymoma.143,152,153 After thymectomy, patients with myasthenia gravis have attenuation of the severity of symptoms over time but not necessarily complete resolution of them.154–156 About 50% of patients with pure red cell aplasia have thymoma, and about 10% of patients with hypogammaglobulinemia have thymoma. Patients with red cell aplasia, hypogammaglobulinemia, and lupus erythematosus appear to have a poorer prognosis.124,143,152,157
Although the degree of tumor invasiveness is strongly related to stage and prognosis of thymoma, there are data to support the prognostic significance of the histologic findings, independent of tumor stage.146,158,159 The historical classification proposed by Marino and Muller-Hermelink categorized thymoma as cortical, mixed, and medullary types.160 Thymomas arising from the epithelial cells of the cortex were classified as cortical thymomas, and those arising from the medullary spindle cells were classified as medullary thymomas. According to the Marino and Muller-Hermelink data, cortical thymomas have a more aggressive course than medullary thymomas and are more likely to be associated with myasthenia gravis. Of note, thymic carcinomas were categorized as a separate entity.
In 1999, the World Health Organization (WHO) published a classification including six subtypes of thymic tumor, based on the relative proportion of epithelial and lymphocytic cells (Box 44-2). This is the most widely accepted histologic classification system, and data have shown that the WHO cell type is an independent prognostic factor.159,161,162,163 A retrospective study of 324 patients found that patients with WHO types A, AB, and B1 had a 100% disease-specific survival rate without RT and therefore did not benefit from adjuvant RT.164 There was no survival difference for cell types B2 and B3 with or without adjuvant RT. Another series, from Reiker and colleagues,165 noted that survival rates of patients with types A, AB, B1, and B2 were better than rates for type B3, and that type C had the lowest OS rates. The prognostic value of the subtype of type B thymomas has been examined by one series that found no significant differences in recurrence or survival rates among the three subtypes of type B, but all patients who recurred had stage III disease, indicating that the Masaoka stage was predictive.166 The risk of myasthenia gravis increases progressively with WHO type from A (~20%) to B3 (~60%).93
Box 44-2 World Health Organization Classification of Thymic Epithelial Tumors
| Type | Pathologic Classification | Prognosis |
|---|---|---|
| A | Medullary thymoma | Benign clinical course |
| Spindle cell thymoma | ||
| AB | Mixed thymoma | |
| B1 | Lymphocyte-rich thymoma | Moderately malignant clinical course |
| Lymphocytic thymoma | ||
| Predominately cortical thymoma | ||
| Organoid thymoma | ||
| B2 | Cortical thymoma | |
| B3 | Epithelial thymoma | |
| Atypical thymoma | ||
| Squamoid thymoma | ||
| Well-differentiated thymic carcinoma | ||
| C | Thymic carcinoma | Highly malignant clinical course |
Tumors that are mixed tend to have an intermediate prognosis.167 The 10-year survival rates are approximately 100% for medullary thymomas, 76% for mixed thymomas, and 45% for cortical and well-differentiated thymomas.167–170 In one series, no medullary and mixed thymomas (types A and AB) recurred, even though 30% had capsular invasion. Organoid and cortical thymomas (types B1 and B2) showed intermediate invasiveness and a low but significant risk of late relapse, even with minimal invasion.29,159
Associated paraneoplastic syndromes occasionally exist with well-differentiated thymic carcinomas.94,95 However, clinical features have not proved useful as prognostic indicators for thymic carcinomas.159 As with thymomas, total resection and stage at presentation are important prognostic factors for thymic carcinoma.18
Diagnostic Workup for Thymic Tumors
The diagnostic workup of a patient with an anterior mediastinal mass begins with a thorough history and physical examination. Particular focus should be given to detect signs and symptoms that may suggest myasthenia gravis, such as fatigue, diplopia, ptosis, and dysarthria. Constitutional symptoms such as fever, chills, and weight loss are more suggestive of lymphoma. Routine screening blood work and chemistry testing may give clues to the presence of associated syndromes. In the case of suspected Cushing’s syndrome, seen in 1% to 2% of patients, a dexamethasone suppression test and urinary cortisol level should be obtained., although some carcinoids can be suppressed, making diagnosis difficult. The differential diagnosis, in addition to thymic lesions, includes germ cell tumors, lymphomas, and thyroid proliferative disorders. Serum alpha-fetoprotein and beta-human chorionic gonadotropin levels should be obtained in young men if a nonseminomatous germ cell tumor is suspected.171–173 Exclusion of extrathymic primary tumors is important because carcinomas and carcinoids are rare in the thymus and may represent metastases.
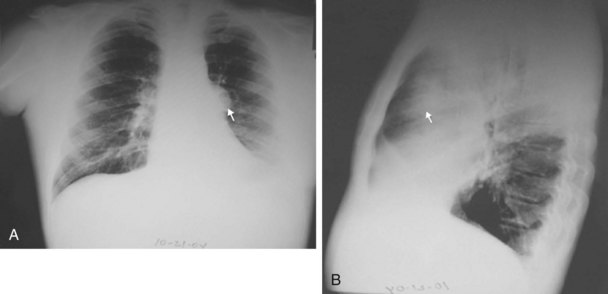
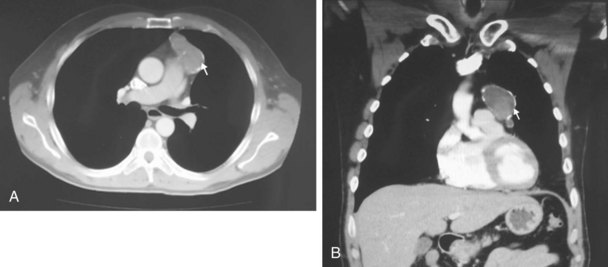
The chest radiograph often demonstrates a mass in the hilar region that may be mistaken for the heart border or pulmonary artery (Fig. 44-2). CT defines174,175 the size, contour, tissue density, and homogeneity of a mediastinal lesion, as well as its relation to or invasion of other mediastinal structures. A CT scan with intravenous contrast dye is preferred to delineate the relationship between the thymoma and surrounding structures and to define the degree of the tumor’s vascularity. Thymic tumors are usually homogeneous, well-demarcated lesions with a round or lobulated shape, and they vary in size and occasionally have calcification176 (Fig. 44-3). The presence of fat planes between the tumor and adjacent structures suggests localized disease. Pleural involvement may be seen in advanced disease. The radiographic presence of both an anterior compartment mass as well as “drop” metastases to the pleura is highly suggestive of the diagnosis. Thymic carcinomas often contain calcifications, cysts, or necrosis on imaging.177 In the absence of symptoms and signs, extensive radiographic imaging is unnecessary. Magnetic resonance imaging (MRI) has not been superior to CT scanning.178 The role of positron emission tomography (PET) has not been established, but early data suggest it can differentiate thymoma from thymic hyperplasia, and high uptake of fluorodeoxyglucose appears to correlate with the degree of tumor invasiveness.179–181
Patients presenting with a thymic mass need a histologic diagnosis before definitive therapy is instituted. CT- or ultrasound-guided fine-needle aspiration biopsy of the mass may be performed to establish the diagnosis preoperatively.182 Such procedures have a sensitivity and specificity of 87% to 90% and 88% to 100%, respectively, for thoracic neoplasms.183 When larger tumor samples are required to distinguish between lymphoma and lymphoid-predominant thymoma, core-needle biopsy provides sufficient specimens with an overall sensitivity of 96% and specificity of 100%.183 Bronchoscopy, video-assisted thoracoscopic surgery, mediastinoscopy, or anterior thoracoscopy may help yield the diagnosis before resection, especially if enlarged lymph nodes are present.172,184,185 The potential risk of breaching the capsule, leading to spillage and seeding of tumor cells during biopsy, has been debated and remains unsettled.136,186,187
Staging
Bergh and associates122 introduced the first clinical staging system for thymoma in 1978. Their staging system was subsequently modified and refined by Masaoka and associates in 1981141 and is the most widely accepted (Box 44-3). It is based on pathologic findings at time of surgery.
Box 44-3
Masaoka Staging System for Thymoma
| Stage | Description |
|---|---|
| I | Macroscopically completely encapsulated with no microscopic detectable capsular invasion |
| II | Macroscopic invasion into surrounding mediastinal fatty tissue or mediastinal pleura or microscopic invasion into the capsule |
| III | Macroscopic invasion into surrounding organs (e.g., pericardium, great vessels, lung) or intrathoracic metastases, or both |
| IVA | Pleural or pericardial implants or dissemination |
| IVB | Lymphogenous or hematogenous metastases |
Adapted from Masaoka A, Monden Y, Nakahara K, Tanioka T: Follow-up study of thymomas with special reference to their clinical stages, Cancer 48:2485-2492, 1981.
A separate, simplified staging paradigm was proposed by Suster and Moran.188 Stage I lesions are localized and encapsulated; stage II lesions are locally invasive; and stage III lesions have nodal, visceral, or distant metastasis. A tumor-node-metastasis (TNM) type of staging system was proposed by Tsuchiya and associates189 but has not been proved clinically, and it is not widely accepted. There is no formal American Joint Committee on Cancer (AJCC) staging system for thymoma.
Treatment
Surgery for Thymoma
Surgery is the mainstay of therapy in resectable cases. However, adjuvant or neoadjuvant radiation therapy plays an important role in subtotally resected or unresectable cases. Many patients who present with symptoms of myasthenia gravis benefit from undergoing a thymectomy.151 Consequently, surgical resection alone for stage II thymomas, in the hands of an experienced team, is a reasonable approach.190,191 In general, surgical resection for thymoma carries low risks of morbidity and mortality; most surgical deaths can be attributed to a myasthenia gravis crisis, but the risk can be minimized with appropriate perioperative management, including plasmapheresis, in selected situations.124,192 After an encapsulated thymoma without associated myasthenia gravis is removed without disturbing the integrity of the capsule, recurrences have been observed, but they are rare.46,60,123,193
Successful surgical treatment of locally invasive thymoma depends on the completeness of resection.194 Consequently, the surgeon should remove as much of the lesion as possible, including surrounding mediastinal fat, possibly to include an extended thymectomy.195 However, resection of an involved phrenic nerve is controversial, and surgeons advocate debulking alone, leaving both phrenic nerves intact if there is bilateral phrenic nerve involvement, because of the respiratory morbidity that would result from resection. When complete resection is not possible, a debulking operation should be considered, because good long-term results can be achieved when such surgery is followed by postoperative radiation therapy.124 Only about one-fourth of patients with stage IVA disease have resectable disease.58 Surgical approaches have varied from discrete resection of pleural metastases to en bloc mediastinal dissection with EPP.196 A few small single-institution series have suggested that EPP may improve local control rates.196–198 The surgeon should delineate the extent of the tumor, specify the areas of invasion, and identify the areas of positive or questionable margins and residual disease with metallic clips to assist future radiation therapy planning.
Radiation Therapy for Thymoma
Although the role of adjuvant irradiation for invasive thymomas has never been tested in a prospective, randomized fashion, there is general agreement that RT is an effective adjuvant therapy for invasive thymomas,199 and most retrospective studies have reported improvements in local tumor control and survival rates after adjuvant irradiation.* However, patients with encapsulated, noninvasive stage I thymoma do not require postoperative RT after complete resection, because the recurrence rate is approximately 0% to 2%.†
For patients with Masaoka stage II disease, there is controversy about whether postoperative RT should be used for completely resected invasive thymomas. A meta-analysis of 22 studies with 592 patients with completely resected stage II or III thymoma found no reduction in recurrence after adjuvant RT,203 but it is difficult to draw firm conclusions from the available data, particularly given the selection bias in retrospective series as to which patients received RT. Berman and colleagues10 reported a single-institution retrospective cohort comparison of 74 stage II thymoma patients after complete surgical resection. Patients who were thought to be at high risk for local recurrence by the operating surgeon and the thoracic oncology team were referred for adjuvant RT. The rate of locoregional recurrence was 8% in patients who were observed versus 0% in the RT group (p = .15). The researchers concluded that the rates of recurrence after complete resection of stage II thymoma were low and that no benefit of postoperative RT was observed in stage II thymoma patients as a group; however, a higher-risk subset of these patients may benefit from adjuvant therapy.10 Although some studies showed no benefit from adjuvant RT,164,190,191,204 the recurrence rates of surgery alone, even with complete resection, may approach 30%.65,143 A SEER study of 901 patients showed a 10% absolute improvement in 5-year OS rates but no statistical difference in cause-specific survival rates in patients with stage II to III disease.204 In cases with gross fibrous adhesions of the tumor to the pleura at the time of surgery or microscopic invasion of the pleura on histologic studies, there is an increased risk for recurrence. Haniuda and colleagues202,206 found that patients with fibrous adhesion to the mediastinal pleura without microscopic invasion benefited most from postoperative radiation therapy. The recurrence rates, with and without adhesion to the mediastinal pleura, were 36% and 0%, respectively. In another study by Monden and colleagues,70 patients with resected stage II thymoma had recurrence rates of 8% and 29%, respectively, with and without adjuvant radiation therapy. Although postoperative radiation therapy may decrease local recurrence, it does not appear to decrease the incidence of subsequent pleural dissemination that may occur outside of radiation fields; in fact, the pleura is the most common site of failure after radiation.202 This may be a reflection of the natural pattern of spread of this disease, with pleural dissemination occurring before, during, or after the time of surgery. Extended irradiation to include the entire pleura significantly increases the normal tissue toxicity and is not routinely employed.
Locally Advanced Thymoma and Palliation
For patients with stage III to IV disease, the evidence supporting the use of postoperative radiation therapy is robust. Urgesi and colleagues140 reported no in-field recurrences in a study of 33 patients with completely resected stage III thymoma treated with postoperative irradiation. Curran and associates65 reported a 53% 5-year actuarial mediastinal relapse rate with stage II and III patients after surgery alone, compared with 0% after total resection and irradiation and 21% after subtotal resection or biopsy and irradiation. Similarly, in a study of 70 patients with Masaoka stage III to IV thymoma, the relapse rate for patients receiving postoperative radiation therapy was reduced from 50% to 20%, and most disease (80%) recurred outside of the irradiated field.207
Preoperative adjuvant radiation therapy has been advocated for unresectable or marginally resectable thymomas.46,71,72,208 Several small studies assessing preoperative radiation therapy for extensive disease found a decrease in tumor burden at the time of surgery, with response rates as high as 80%, and described a theoretical decrease in the potential for tumor seeding during surgery.65,124,209,210 Onuki and colleagues210 found that a dose of 12 to 20 Gy given to 21 patients with stage III thymomas resulted in a 76% response rate, with more aggressive WHO histologic subtypes having a less robust volume reduction. These series demonstrated that preoperative irradiation facilitated total or subtotal resection of the invasive thymoma mass by reducing the tumor volume.208
Primary radiation therapy alone as the definitive treatment has been advocated in nonsurgical candidates or patients with unresectable advanced disease. In a study of 12 patients who presented with unresectable tumors and were treated with primary radiation therapy, 7 patients were alive 1 year 8 months to 5 years 1 month later.211 Similar outcomes were reported for 31 patients with unresectable stage III or IV disease.212 Five-year survival rates of 53% to 87% and 10-year survival rates of 44% were reported for patients with advanced thymoma receiving irradiation after biopsy only or incomplete resection.213,214 Urgesi and associates215 reported the use of radiation therapy alone in 21 patients with intrathoracic recurrences of thymoma. The 7-year survival rate of 70% was similar for those treated with irradiation alone and those treated with surgery and adjuvant therapy. However, the retrospective nature of these studies, small number of patients, differing amounts of clinical disease, and variations in radiation doses and techniques are significant confounding variables.
Chemotherapy for Thymoma
In general, thymomas are chemosensitive tumors, and chemotherapy is reserved for locally advanced or metastatic disease. Numerous case reports and small series have reported antineoplastic activity for multiple and single agents in the treatment of patients with advanced thymoma.* However, there are no reported prospective, randomized trials comparing different chemotherapeutic agents.233 Platinum-based regimens have commonly been used over the past decade, with response rates ranging between 24% and 100%, and response rates of more than 50% have been found consistently with the application of cisplatin-based combination chemotherapy.234 Some of the commonly employed combinations are cisplatin, doxorubicin, and cyclophosphamide (PAC), with reported overall responses in excess of 70%,235–237 and cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC).238 In a study by Fornasiero and colleagues,222 37 patients with stage III or IV thymoma were treated with ADOC, with an overall response rate of 92% and a 43% complete response rate. Loehrer and associates235 studied 23 patients with localized but unresectable thymomas treated with PAC followed by radiation therapy. The overall objective response rate was 70%, with five complete responders.239 The estimated 5-year disease-free survival rate was 54%, with a median survival time of 93 months. Multivariate analysis demonstrated that chemotherapy was associated with an improvement in OS rates for patients with stage III and IV disease. Similar results with combination PAC have been reported in Taiwan; all responders had overexpression of the topoisomerase 2α gene, but patients with no detectable expression progressed with treatment.239
Combined-Modality Therapy for Thymoma
The role of chemotherapy as an adjuvant therapy after resection has not been established, but there is anecdotal evidence that combining chemotherapy with irradiation with or without surgery can be effective with acceptable toxicity in selected cases.230,240,241,242 It has also been shown that neoadjuvant chemotherapy and then surgery, followed by additional chemotherapy and irradiation, may improve the resectability and survival rates of the patients with locally advanced disease.241
In a phase II study of 22 patients with unresectable thymoma treated with induction PAC resection, adjuvant irradiation, and consolidative chemotherapy, there was a 77% response rate to chemotherapy, a 95% 5-year OS rate, and a 79% 7-year OS rate.237 A trial from the Japanese Clinical Oncology Group reported on 23 patients with unresectable stage III thymoma treated with weekly dose-dense cisplatin, vincristine, doxorubicin, and etoposide and found no deaths from toxicity, but only 57% were able to complete chemotherapy as planned.243 Thirteen of the patients were able to undergo resection, with nine R0 resections. With a 62% response rate, efficacy of the dose-dense regimen was no better than with conventional chemotherapy.235,237
In a study by Macchiarini and colleagues,230 all seven patients with stage III invasive thymoma treated with neoadjuvant cisplatin, epirubicin, and etoposide were able to undergo surgical resection. Four had complete resections, and three had incomplete resections. Shin and colleagues240 reported that 9 of 11 patients with unresectable stage III and IV thymoma were able to undergo complete resection after induction chemotherapy consisting of cyclophosphamide, doxorubicin, and cisplatin. All nine patients were given additional postoperative radiation therapy and chemotherapy. Of these patients, seven were disease free at a median follow-up time of 43 months. Wright and associates244 reported on 10 patients with stage III to IVA thymoma treated neoadjuvantly with two cycles of cisplatin and etoposide and concurrent irradiation followed by surgery. Eight patients had R0 resections, and the 5-year survival rate was 69%.
Results of Thymoma Treatment
There are no large prospective, randomized, phase III clinical trials to evaluate the efficacy of primary or adjuvant radiation therapy in patients with invasive thymoma, but multiple retrospective reviews have suggested that RT reduces recurrence rates and improves outcomes for incompletely resected stage II to III thymomas. The role of postoperative RT for completely resected stage II to III thymomas is controversial. The local control rates after a complete resection and adjuvant radiation therapy have ranged from 65% to 100%, and rates are lower for incomplete resection and radiation therapy.* Curran and coworkers65 reported a retrospective study of patients with stage II or III thymoma. Twenty-six percent (20 of 78) of the patients treated with surgery alone developed local recurrences, compared with 5% (2 of 43) of similar patients who had received postoperative radiation therapy. There was no significant difference in relapse rates or survival rates between patients undergoing biopsy and radiation therapy and those having subtotal resection and radiation therapy. The investigators reported a 5-year actuarial mediastinal relapse rate of 53% for patients treated with total resection alone, compared with no relapses in patients who received postoperative irradiation after total resection. Contrary to most studies, Kondo and Monden18 reported a multi-institutional, retrospective study of 1320 patients with thymic epithelial tumors and found no significant difference in the recurrence rates between two groups with stage II or III thymoma, one of which underwent surgery alone and the other surgery plus adjuvant irradiation. This observation may reflect the fact that most of the patients underwent complete resection (100% with stage II disease and 85% with stage III disease); as has been pointed out by the study authors, the recurrence rates they found were lower than in previous reports.
Postoperative radiation therapy has resulted in improved survival rates for patients with invasive thymomas after complete and incomplete resections.233 In a study of 141 patients with thymoma, Nakahara and associates125 reported a 5-year survival rate of 92% for patients with stage II disease and 88% for patients with stage III disease who were treated with postoperative irradiation. Patients undergoing radical surgery with complete resection before adjuvant radiation therapy often had substantially better local control and 5-year survival rates compared with those undergoing biopsy alone or limited tumor resection.124,125,209,246
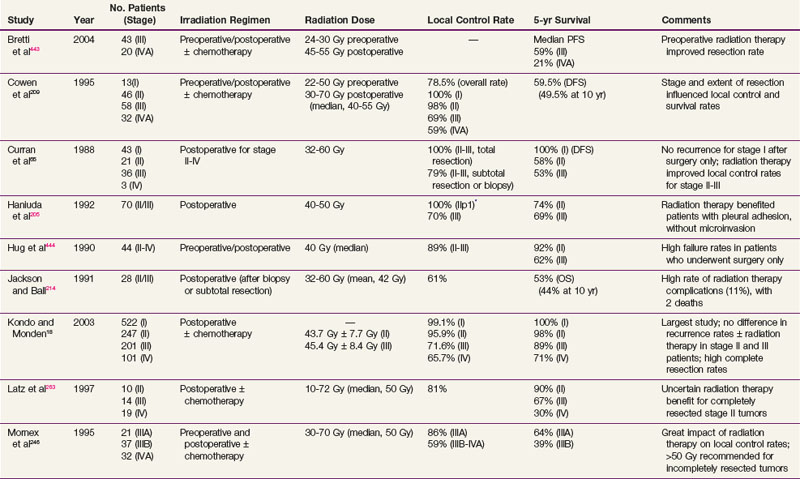
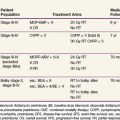
After complete resection, invasive thymomas still tend to carry a poorer prognosis than noninvasive tumors.47,233,247 Survival rates continue to fall with long-term follow-up, and 10-year survival rates may be considerably lower.149 The 5-year and 10-year survival rates for well-encapsulated thymomas without invasion are more than 90%, and for invasive thymomas, the rates range from 30% to 70%† The 5-year survival rates according to Masaoka stage are 83% to 100% for stage I disease, 86% to 98% for stage II disease, 68% to 89% for stage III disease, and 50% to 71% for stage IV disease.18,68,141,248 The approximate 10-year survival rates are 80%, 78%, 47%, and 30%, and the 15-year rates are 78%, 73%, 30%, and 8% for stages I to IV, respectively.68 Table 44-1 provides a summary of treatment results.
Anecdotal studies have demonstrated good local control of thymomas treated with primary irradiation. Of 23 patients treated with irradiation alone, 8 had complete regression, 10 had partial regression, and 5 had no regression of disease.46 In a report by Marks and colleagues,249 tumor was controlled in all nine cases treated with megavoltage irradiation. The average follow-up was 5.5 years (minimum, 30 months). Ariaratnam and associates250 observed tumor control in 8 of 11 patients with malignant thymoma with a minimum follow-up of 2 years. Two of the three patients who died had received only 30 Gy in 3 weeks to the mediastinum. Overall, irradiation as monotherapy may result in local control rates of approximately 65% and 5-year survival rates of 40% to 50%.213,214,233
In patients with mostly advanced thymoma, the results of combination chemotherapy with or without surgery or irradiation are encouraging, with an overall response rate of approximately 60%.222,224,236 All patients who achieved a pathologic complete response had received cisplatin-containing regimens. An intergroup study of 26 patients with unresectable disease treated with two to four cycles of PAC followed by RT (54 Gy) in those without progression was well tolerated. There was a 70% response rate to induction chemotherapy and a median survival rate of 93 months, and a 53% 5-year OS rate, which compares well with neoadjuvant chemotherapy followed by resection.229
Treatment of Thymic Carcinoma
Although the optimal treatment of thymic carcinoma is unclear, surgical extirpation remains the cornerstone of therapy, and in most published studies, surgery has been followed by adjuvant radiation therapy.17,64,251–253 Because many series are gathered over decades, it has been difficult or impossible to control for the changes in pretreatment tumor imaging, surgical techniques, and thoracic radiation therapy planning and delivery, all of which may contribute to the inconsistent results in the literature. A prescriptive dose range has yet to be identified, with most studies using 40 to 70 Gy with a standard fractionation scheme (1.8 to 2 Gy per fraction), but in general, doses of 45 to 55 Gy are used in the adjuvant setting, whereas 60 Gy or more is used for definitive irradiation. Although a survival benefit has not been seen, there is a trend toward improved survival and local control rates with adjuvant therapy.17,252 No dose-related survival advantage has been established, although a relationship between dose and local control rates has been demonstrated.254
In a series of 26 patients treated with surgery and postoperative irradiation, Hsu and associates252 observed a 77% 5-year OS rate, with respective 82% and 66% survival rates for completely resected and subtotally resected cohorts. With a median dose of 60 Gy (range, 40 to 70 Gy), an excellent 5-year local control rate of 91% was observed. For a cohort of 40 patients receiving definitive or adjuvant radiation therapy, Ogawa and associates17 reported an absence of local recurrence for those with complete resection and radiation doses greater than 50 Gy. Kondo and Monden18 reported the largest retrospective, comparative study and found no statistically significant survival benefit from the addition of adjuvant irradiation to surgical resection, although the study authors stipulated that no definitive conclusions could be made regarding the role of adjuvant radiation therapy because of subgroup sample size limitations and the retrospective nature of the study.
Although local control rates are increased with irradiation, a survival benefit remains to be demonstrated. For patients with any question of clinical resectability, neoadjuvant platinum-based chemotherapy is a reasonable treatment consideration.255 Table 44-2 summarizes some of the treatment results of thymic carcinoma.
Treatment of Thymic Carcinoid
Complete surgical resection is the preferred method of treatment, although recurrence and distant metastases are common.18,256,257 A SEER analysis of 160 patients found that the median survival time was 79 months in patients who had surgical resection compared with 26 months in those who did not have surgery Patients who received irradiation had a worse OS rate, but patients who received irradiation were more likely to have advanced disease.257 In a study of 40 patients, the recurrence rate was 64%, even though 35 of the 40 patients had a complete resection,18 and the 5-year survival rate was 84%. Despite a lack of conclusive evidence, incomplete resections followed by irradiation or chemotherapy, or both, may improve local control rates without significantly increased morbidity and mortality rates.55,129,258–260 However, distant metastases occur in approximately 30% of patients.260 The long-term prognosis is poor, with an overall 5-year survival rate of 0% to 31%.256,261
Techniques of Irradiation
High-energy (>10-MV) x rays usually are preferred for irradiation. A CT scan is indispensable for adequate treatment planning. Clips placed at the time of surgery denoting the extent of resection in completely resected tumors or outlining regions of residual disease are useful in guiding postoperative irradiation. The planning target volume (PTV) should include the gross tumor volume (GTV) and the thymus, if any, and the tumor bed with a 1.5- to 2-cm margin. A four-dimensional breathing scan, if available, should be used to determine tumor motion. This information can be used to create an internal target volume (ITV) or to plan for radiation-coordinated breathing control, wherein the patient is treated only during a particular phase of the breathing cycle. Although the latter approach requires significant patient training and cooperation, it can provide superior sparing of normal lung.262 Depending on histologic and surgical findings, areas of suspicious subclinical disease and regional lymphatics are commonly included. However, treatment of the entire mediastinal and supraclavicular nodal basin prophylactically is not advised, because of the low incidence of lymph node involvement and a higher normal tissue complication rate when large target volumes are used.235,245,263,264 A SEER database analysis of 1334 patients from 1973 to 2005 by Fernandes and colleagues265 found no increase in long-term cardiac mortality rates or rates of secondary malignant tumors for patients treated with RT compared with patients treated with surgery alone.
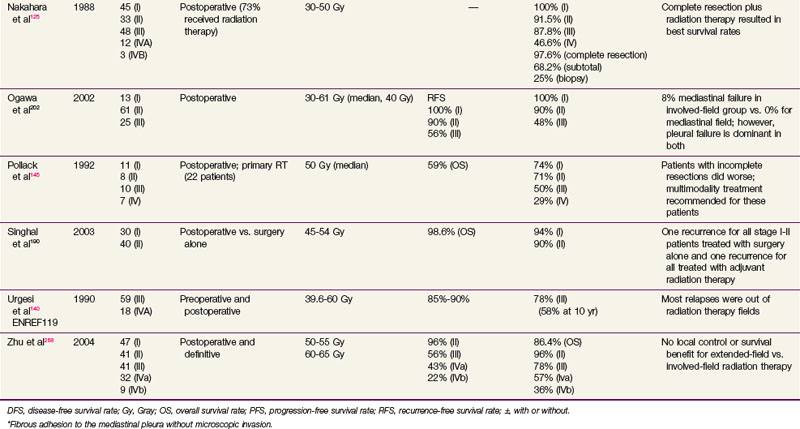
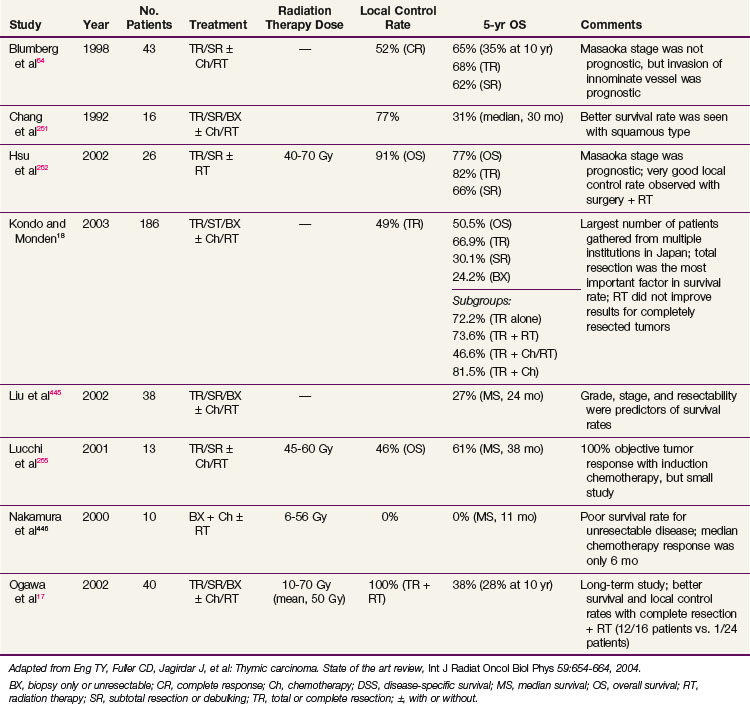
Conventional port arrangements may include a single anterior port, two opposed anteroposterior ports (weighted 2 : 1 or 3 : 2), wedged-pair techniques, and other multiple-field arrangements. Given the advanced technical capabilities available, three-dimensional conformal RT (3DCRT) or intensity-modulated radiation therapy (IMRT) should be considered to provide dose homogeneity and allow better sparing of normal critical structures. Figure 44-4 shows a postoperative radiation therapy field with multileaf collimators shaping the surgical tumor bed. Figure 44-5 shows a 3DCRT field with IMRT. The isodose lines more closely conform to the shape of the PTV. The risks of side effects depend on normal tissue tolerance, surrounding critical organs, prior treatments, irradiation dose, other concurrent therapies, and the general health of the patient.
Various dose and fractionation schemes for thymoma are reported in the literature, and it is unclear if there is a dose response.233,266 Although one retrospective study did not find any relationship between radiation dose and local control rates,209,254 others have found that radiation dose was a significant prognostic factor for local control rates, with good results reported when doses higher than 40 Gy were used for subclinical or microscopic disease.246,267 Retrospective data from Zhu and co-workers268 found a 5-year survival rate of 82% in patients given a dose greater than 50 Gy, compared with 70% for doses less than or equal to 50 Gy. The most important prognostic factors were Masaoka stage and extent of resection.
Postoperative doses of 45 to 55 Gy given by conventional fractionation have been used effectively in most cases.* The dose to the spinal cord should be limited to 45 Gy and can be accomplished using oblique mediastinal fields or IMRT. For patients with gross residual disease after resection or unresectable disease, doses of 60 Gy or more are probably required,145,212,246 but higher doses correlate with higher risks of complications.269 Sophisticated treatment planning with IMRT may facilitate delivery of high doses to gross tumor while respecting dose limits to organs at risk.270
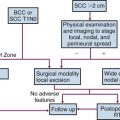
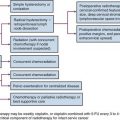
Treatment Algorithm and Controversies
Pulmonary Carcinoid Tumors
Neuroendocrine lung tumors have a spectrum of clinical behaviors and include typical carcinoids (the most indolent type), atypical carcinoids, large cell neuroendocrine tumors, and small cell lung carcinomas (the most aggressive type).271 The term carcinoid (from the German karzinoide) was originally defined as a carcinoma-like lesion without malignant characteristics by Oberndorfer in 1907.272 Pulmonary carcinoids used to be known as bronchial adenomas because they were believed to be benign, but they are now recognized as malignant because of their potential to metastasize. Pulmonary or bronchial carcinoid tumors are typically low-grade malignant neoplasms that arise from neuroendocrine cells of the amine precursor uptake and decarboxylation (APUD) system that have migrated from the embryonic neural crest. These cells are the Kulchitsky (enterochromaffin) cells, which are located in the basal layer of bronchial epithelium.273,274 Approximately 25% of carcinoids are located in the respiratory tract, which is the second most common site after the gastrointestinal tract. Pulmonary carcinoids are frequently centrally located and confined to the main or lobar bronchi.275 These tumors are rare, and their biologic behavior mostly depends on their histologic characteristics.
Etiology and Epidemiology
The cause of pulmonary carcinoid is unknown, and no environmental risk factors have been identified. Carcinogens that are associated with lung cancer have not been consistently associated with pulmonary carcinoids,276 although a few studies revealed a higher frequency of smokers with atypical carcinoids.277–279 Some of the neoplastic cells express mutant retinoblastoma (RB1) or mutant TP53 gene products, and they show occasional loss of heterozygosity, especially in atypical carcinoids.280 Most familial pulmonary carcinoids have been reported in patients with multiple endocrine neoplasia type 1 (MEN 1). In cases of atypical and typical lung carcinoids, there is a characteristic allelic loss within the region of 11q-13, which harbors the MEN1 gene, a tumor suppressor gene.281–285 Other frequently detected genetic alterations in lung carcinoids, especially atypical carcinoids, include losses of 3p, 5p, 9p, 10q, and 13q.283,284 Ki-67 expression in more than 5% of nucleoli is a poor prognostic factor for survival.286
The annual incidence of carcinoid tumors is one or two cases per 100,000 people in the United States.2,276 For bronchopulmonary carcinoids, the reported incidence is 0.6 per 100,000 people.3 Carcinoid tumor of the lung represents 1% to 2% of all lung malignant tumors, with an equal frequency of these tumors occurring in men and women276; approximately 3500 new cases were reported in 2004.287,288 Bronchial carcinoids are the most common primary lung tumor in children, typically presenting in late adolescence. Typical carcinoids are about four times more common than atypical carcinoids. Data from the SEER program of the National Cancer Institute have shown a trend of relative increase of pulmonary carcinoids.3,276
Prevention and Early Detection
There is no known preventive measure for pulmonary carcinoids. Carcinoids are detected like other space-occupying tumors. The most common findings on a chest radiograph include a hilar mass with occasional atelectasis. Functioning carcinoids are suspected on the basis of the symptoms and signs, and the diagnosis is confirmed by demonstrating increased urinary excretion of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA). Thyroid transcription factor-1 is expressed in 80% of metastatic pulmonary carcinoids but not in intestinal carcinoids, and it may be of value in the workup and diagnosis of pulmonary carcinoids.289,290 Sometimes, localization of the tumor may require an extensive evaluation, including laparotomy. If hepatic involvement is suspected, a specific liver scan may be sufficient to demonstrate metastases.
Biologic Characteristics and Molecular Biology
Carcinoid tumors often occur sporadically and are thought to arise from neuroendocrine cells, which are present in a wide range of organs. The WHO classification of lung neuroendocrine tumors describes two different subtypes of carcinoids, typical and atypical carcinoids, with distinctive clinical behaviors and different prognoses.288,291 Based on the criteria proposed by Travis and associates,271,291 the spectrum of neuroendocrine tumors also includes large cell neuroendocrine carcinoma and small cell lung carcinoma.
Although most pulmonary carcinoids are nonfunctional, some have the ability to synthesize and secrete a variety of physiologically active substances, leading to paraneoplastic syndromes, including carcinoid syndrome, Cushing’s syndrome, and acromegaly.274 Serotonin (5-hydroxytryptamine [5-HT]) is one of the frequently encountered vasoactive substances released by carcinoid tumors. Other substances, including corticotropin, histamine, dopamine, substance P, neurotensin, prostaglandins, and kallikrein, have been reported.292–297
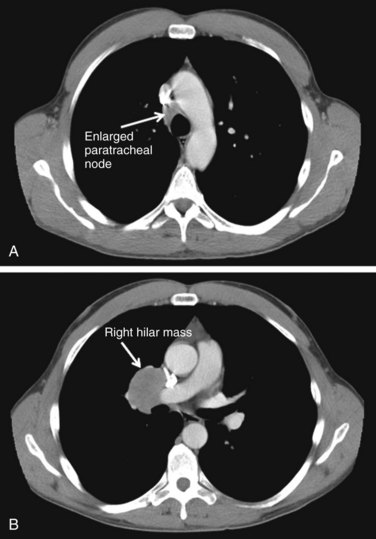
The biologic behavior of these tumors varies depending on the histologic characteristics. Typical carcinoid tumors of the lung generally have a better prognosis than other primary lung cancers, with a 5-year survival rate greater than 90%. For atypical carcinoids, 5-year survival rates range from 25% to 75%, depending on the series.298–303 A population-based study of over 1400 patients from several European countries showed a 5-year OS rate of 78% for pulmonary carcinoids as a whole.304 Although typical carcinoids have an indolent clinical course, atypical carcinoids behave more aggressively, with nodal involvement in 20% to 60% of cases and a higher rate of distant metastases. Figure 44-6 shows a right-sided hilar atypical carcinoid tumor with associated mediastinal adenopathy. The recurrence rates and stage at presentation frequently are higher than for typical carcinoids. In atypical carcinoids, there is a high frequency of molecular alterations and inactivation of tumor suppressor genes, such as the TP53, RB1, CDKN2A (previously designated p16), and CDKN2D (previously designated p19) genes. The proliferation rates, as assessed by MIB-1 and Ki-67, are also higher than for typical carcinoids.288,305,306
Pathology and Pathways of Spread
Carcinoids are thought to be derived from different embryonic divisions of the gut and are classified accordingly. Foregut carcinoids are often found in the lungs, bronchi, and stomach, and midgut and hindgut tumors are typically seen in the small and large intestines. Histologically, they may stain positive with silver or Grimelius stains, cytokeratin 7 and 11, neuron-specific enolase, synaptophysin, carcinoembryonic antigen, and chromogranin; there is no marker exclusive to carcinoids, however.289,307,308 Microscopically, they are characterized by small uniform cells and the presence of numerous membrane-bound, neurosecretory eosinophilic granules containing a variety of hormones and biogenic amines in the cytoplasm. Most pulmonary carcinoids (70% to 90%) are typical carcinoids or well-differentiated neuroendocrine tumors, which are characterized by small cells with well-rounded nuclei; atypical carcinoids (10% to 30%), or poorly differentiated neuroendocrine tumors, have malignant histologic features of increased nuclear atypia with high mitotic activity (>2 mitoses per 10 high-power fields), lymphovascular invasion, nuclear pleomorphism, and areas of necrosis.291,308–311
It is sometimes difficult to establish the specific diagnosis of carcinoid because of small biopsy samples or to differentiate atypical carcinoid from small cell lung carcinoma without immunohistochemical tests.291,312–314 Well-differentiated carcinoids are often indolent, with a relatively low risk of nodal or distant metastasis of 3% to 15%.287,311,315–317 Atypical carcinoid may have an aggressive clinical course, with frequent organ metastases.280,318,319 The risk of nodal metastasis, frequently to mediastinal lymph nodes, is 30% to 57%.287,320,321 Distant metastases occur in more than 20% of patients.287 The most common sites of distant metastasis include liver, bone, adrenal glands, brain, skin, and soft tissue.288,299,322–324
Clinical Manifestations, Patient Evaluation, and Staging
Most patients with pulmonary carcinoids present in their 50s.2,276,287,319 Carcinoids in children are rare.325 Between 70% and 80% of these tumors are located centrally near the hilum within the tracheobronchial tree and may produce a variety of clinical symptoms and neuroendocrine manifestations at the time of diagnosis.326,327 About 25% of patients present with symptoms such as recurrent pneumonia, cough, hemoptysis, or chest pain. Carcinoids are vascular tumors and can bleed secondary to bronchial irritation. Hyperparathyroidism may be seen. At least 50% of pulmonary atypical carcinoid tumors present in the periphery of the lung.328 Patients with peripheral lesions may be asymptomatic, and their lesions are usually discovered incidentally.300,329
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree