Chapter 56 Cervical Cancer
The incidence of and mortality rates from invasive cervical cancer have steadily declined in the United States over the past five decades, in part because of the successful implementation of screening programs that detect many cancers at a preinvasive stage. Unfortunately, cytologic screening programs have failed to comprehensively penetrate the population, with many medically underserved or uneducated communities at risk. Invasive cervical cancer continues to be a major public health problem internationally. In the United States, 60% of women with newly diagnosed invasive cervical cancers have not had a Papanicolaou (Pap) smear for 5 years or longer.1
Epidemiology and Etiology
In the United States, cervical cancer is responsible for about 2% of the cancer deaths in women, with about 12,200 new cases of invasive disease and 4210 deaths in 2010.2 Worldwide, invasive cervical cancer is the third most common malignant tumor in women (after breast and colorectal cancers) and accounts for nearly 371,000 cases and 190,000 deaths per year.3 Incidence rates range from as low as 3 to 4 per 100,000 in Israel to more than 80 per 100,000 in Recife, Brazil.4 Presumably, this wide range reflects a variation in epidemiologic risk factors that is compounded by the lack of adequate screening programs in poor communities. The relatively high international mortality rates may also reflect the greater number of women who present with advanced disease when screening programs and patient education are inadequate and access to medical resources is limited or nonexistent. Because of the lack of cancer registries, it is likely that reported incidence and mortality rates grossly underestimate the magnitude of the problem.
Risk factors for the development of carcinoma of the cervix and its intraepithelial precursors follow a pattern typical of sexually transmitted diseases. These include first coitus at a young age, multiple sexual partners, a history of other sexually transmitted diseases, and high parity.5 Weaker associations have been suggested with cigarette smoking and the use of oral contraceptives. In contrast, the risk of developing cervical cancer is low in women who are nulliparous or virginal. Among women with only one lifetime sexual partner, past and current high-risk sexual behavior by the male partner has a substantial role in the development of cervical carcinogenesis.6 Conversely, male circumcision is associated with a reduced prevalence of penile HPV infection and a reduced risk of cervical cancer in the current sexual partners.7
Until recently, the reasons for these associations were only the subject of speculation. Today, however, the epidemiologic findings, combined with the results of molecular studies (discussed in detail later), are sufficient to identify sexually acquired human HPV infection as the primary etiologic agent in the development of most cervical cancers.8,9
Although the epidemiology of cervical adenocarcinoma is somewhat less well understood, studies also reveal the presence of HPV DNA in most cases.10 It is interesting that in the United States, the absolute and relative incidence of adenocarcinoma appears to have increased in contrast to the steady decrease in the rate of invasive squamous carcinoma. This may reflect, in part, differences in the effectiveness of cytologic screening in detecting adenocarcinoma at a preinvasive stage.11 Some investigators have implicated oral contraceptives as a possible risk factor for adenocarcinoma, but this remains controversial.12
Clear cell carcinoma, a rare form of cervical and vaginal adenocarcinomas, has been clearly linked with prenatal exposure to diethylstilbestrol (DES), a drug that was used to prevent miscarriages in the 1940s and 1950s.13 Prenatal DES exposure arrests development of the transformation zone in the upper third of the vagina, which accounts for the location of these lesions. The average age at which cancer in DES-exposed patients was diagnosed was 19 years, much younger than the average age of patients with newly diagnosed squamous carcinoma or non–DES-related adenocarcinoma. New diagnoses of DES-related clear cell carcinoma have declined now that the youngest cohort of DES-exposed patients has passed the age of peak incidence.
Prevention and Early Detection
Although there have been significant changes in the fields of radiation and gynecologic oncology, the dramatic decrease in cervical cancer mortality from the 1940s to the 1980s is primarily due to the success of mass screening programs.14,15 The cervix is an ideal target for cancer screening because of its accessibility, the long average time interval from the initial DNA insult to the development of invasive cancer, and the high cure rate with appropriate treatment of preinvasive and early invasive lesions.
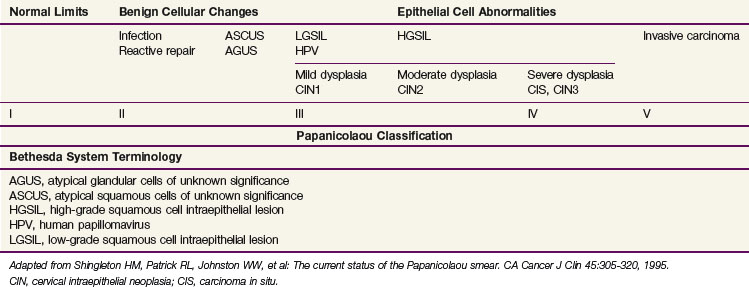
Squamous cell carcinomas originate at the squamocolumnar junction (transformation zone) of the cervix. Invasive lesions are frequently associated with adjacent preinvasive or in situ disease (low-grade squamous cell intraepithelial lesion [LGSIL] or high-grade squamous cell intraepithelial lesion [HGSIL]) (Table 56-1).
Longitudinal studies suggest a long average time to progression from HGSIL to invasive disease. Peterson16 described 127 patients with carcinoma in situ (CIS) (HGSIL) who were followed for at least 3 years. At the end of 10 years, invasive carcinoma had developed in about 30%. Koss and colleagues17 observed spontaneous regression of 25% in 67 patients with untreated CIS (HGSIL) who were observed for 3 years. In a later analysis of this series, the authors reported that invasive lesions had developed in 40% of the original 67 patients.18 In another longitudinal study of patients with untreated CIS (HGSIL), Kottmeier19 observed progression to invasive carcinoma in 71% and 80% of patients followed for 12 and 30 years, respectively.
Longitudinal studies have also demonstrated a relatively long time between the diagnosis of dysplasia and the development of HGSIL. One large prospective study reported mean times for progression to HGSIL development of 58, 38, and 12 months for patients with mild, intermediate, or severe dysplasia, respectively.20 These results are consistent with the finding that the mean age of women diagnosed with cervical intraepithelial neoplasia (HGSIL) is about 16 years younger than that of women diagnosed with invasive carcinoma.21
Although a number of classifications have been used, the Bethesda system is currently the most widely accepted method of categorizing cervical cytologic specimens in the United States.22 The relationship between this system and earlier classification systems is outlined in Table 56-2.
TABLE 56-2 International Federation of Gynecology and Obstetrics Staging of Carcinoma of the Cervix
| Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded). |
| Stage IA | Invasive cancer identified only microscopically. All gross lesions, even with superficial invasion, are stage IB cancers. |
| Invasion is limited to measured stromal invasion with a maximum depth of 5 mm and no wider than 7 mm. (The depth of invasion should not be more than 5 mm taken from the base of the epithelium, either surface or glandular, from which it originates. Vascular space involvement, either venous or lymphatic, should not alter the staging.) | |
| Stage IA1 | Measured invasion of stroma no greater than 3 mm in depth and no wider than 7 mm. |
| Stage IA2 | Measured invasion of stroma greater than 3 mm and no greater than 5 mm in depth and no wider than 7 mm. |
| Stage IB | Clinical lesions confined to the cervix or preclinical lesions greater than IA. |
| Stage IB1 | Clinical lesions no greater than 4 cm in size. |
| Stage IB2 | Clinical lesions greater than 4 cm in size. |
| Stage II | The carcinoma extends beyond the cervix but has not extended onto the pelvic wall; the carcinoma involves the vagina but not as far as the lower third. |
| Stage IIA | No obvious parametrial involvement. |
| Stage IIA1 | Clinical lesions no greater than 4 cm in size. |
| Stage IIA2 | Clinical lesions greater than 4 cm in size. |
| Stage IIB | Obvious parametrial involvement. |
| Stage III | The carcinoma has extended onto the pelvic wall; on rectal examination there is no cancer-free space between the tumor and the pelvic wall; the tumor involves the lower third of the vagina; all cases with a hydronephrosis or nonfunctioning kidney should be included, unless they are known to be due to another cause. |
| No extension onto the pelvic wall but involvement of the lower third of the vagina. | |
| Stage IIIA | Extension onto the lower third of the vagina. |
| Stage IIIB | Extension onto the pelvic wall or hydronephrosis or nonfunctioning kidney. |
| Stage IV | The carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum. |
| Stage IVA | Spread of the growth to adjacent organs. |
| Stage IVB | Spread to distant organs. |
From Pecorelli S, Zigliani L, Odicino F: Special communication. Revised FIGO staging for carcinoma of the cervix, Int J Gynecol Obstet 105:107-108, 2009.
The American College of Obstetricians and Gynecologists (ACOG) recommends that cervical cancer screening should begin at age 21 years regardless of age of onset of sexual intercourse. Cervical cytologic screening is recommended every 2 years for women aged 21 to 29 years, with either conventional or liquid-based methods. Women aged 30 years and older with three consecutive cervical cytologic test results may be screened every 3 years. Women with human immunodeficiency virus (HIV) infection, those who are immunosuppressed, those who have been exposed in utero to DES, or those who have been previously treated for cervical intraepithelial neoplasia (CIN) stage II or III or cancer may require more frequent screening. HPV DNA testing may be used as an adjunct or even a replacement for cytologic screening in women older than 30 years; women in this age-group with both negative cytologic and negative HPV DNA test results may be screened every 3 years. Cytologic screening may be discontinued in women after hysterectomy for benign indications (with no prior history of high-grade CIN) or at age 70 years if three consecutive cytologic screening test results have been negative and no abnormal test results have been seen in the previous 10 years.23 As compared with annual screening, mathematical modeling predicts the excess risk of cervical cancer with less frequent screening intervals to be approximately 3 in 100,000.24
Despite the proven efficacy of cytologic screening, many women remain unscreened. In the United States, 50% of women with newly diagnosed invasive cervical cancer have never had a Pap smear and another 10% have not had a Pap smear in 5 years.9 Women who tend to be underscreened in the United States fall into groups of postmenopausal, uninsured, ethnic minority, and, especially, elderly black and poor women in rural areas. In the United States, about 25% of the cervical cancer cases and 41% of the deaths occur in women who are aged 65 years or older. In addition, nearly 50% of women older than 60 years have not had a Pap smear in 3 years, even though many have seen a physician for other medical reasons.
Pathology and Pathways of Spread
Eighty percent to 90% of cervical cancers are squamous cell lesions. Squamous cell neoplasms are frequently subcategorized as either large cell keratinizing, large cell nonkeratinizing, or small cell carcinomas.25,26 The latter should not be confused with anaplastic small cell carcinomas, which have histologic features that resemble small cell neuroendocrine neoplasms of the lung and tend to have a particularly aggressive clinical course.27,28
The frequency of primary cervical adenocarcinoma is about 10% to 20%, but it appears to have been increasing recently, particularly in younger women.29,30 Cervical adenocarcinomas can originate high in the endocervical canal and can be missed with standard cytologic screening collection methods. The perceived increased frequency of cervical adenocarcinomas may be attributed to a decreased incidence of squamous cell carcinomas in the screened population without a concomitant decreased incidence of cervical adenocarcinomas. Whether conventional screening methods are insensitive to the detection of adenocarcinoma precursor lesions or whether such precursor lesions progress more quickly to invasive disease has not been determined. Nonetheless, women who participate in a cytologic screening program and develop cervical adenocarcinoma are more likely to have early-stage disease than an unscreened cohort. Most cervical adenocarcinomas are mucinous with features suggestive of endocervical glandular epithelium; about 20% demonstrate other müllerian neoplastic patterns. Others may be adenosquamous in type.
The cervix is richly supplied with lymphatics organized into three anastomosing plexuses that drain the mucosal, muscularis, and serosal layers.31 The most important lymphatic collecting trunks exit laterally from the uterine isthmus in three groups31,32: the upper branches originate in the anterior and lateral cervix and follow the uterine artery, the middle branches drain to the deeper hypogastric (obturator) nodes, and the lowest branches drain posteriorly to the inferior and superior gluteal, common iliac, presacral, and subaortic lymph nodes.
The incidences of pelvic lymph node involvement for patients with FIGO stages IB, IIB, and IIIB cervical cancer are approximately 15%, 30%, and 50%, respectively.33–36 The incidence of para-aortic lymph node metastasis also increases with tumor stage; about 5%, 20%, and 30% of patients with FIGO stage IB, IIB, and IIIB disease, respectively, have para-aortic lymph node metastasis at diagnosis.33 For each stage, the risk of lymph node involvement is further correlated with tumor size.37,38
Hematogenous metastases are rarely detectable at diagnosis, and recurrent pelvic disease is seen in about two thirds of patients who relapse after treatment. Distant metastases are also a common feature of disease relapse, however. In a study of disease relapse patterns from the Mallinckrodt Institute of Radiology, Fagundes and associates39 reported 10-year actuarial rates for distant metastasis of 16%, 31%, 26%, and 39% for patients treated with radiation for FIGO stages IB, IIA, IIB, and III disease, respectively. These may well be underestimated if pelvic disease is detected as the first site of relapse because systematic radiologic evaluation may not be performed. The most common site of extrapelvic metastasis was the lungs, followed by the para-aortic lymph nodes. Although the lumbar spine has been reported to be a relatively frequent site of skeletal metastasis, studies that include CT scanning suggest that patients who appear to have had metastases isolated to the lumbar spine may actually have had direct tumor extension from para-aortic disease.40
Prognostic Factors
With the exception of distant hematogenous metastases, the presence of retroperitoneal nodal involvement represents the most significant negative prognostic factor in patients with cervical cancer.34,41 The evaluation of other prognostic factors, however, is to a great degree predicated on whether patients have early-stage tumors that are often managed surgically or more locally advanced cancers that have received definitive chemoradiation. The availability of a hysterectomy specimen for full pathologic review allows finer assessment of primary associated and extrauterine risk factors, although these factors may be superseded in larger tumors with obvious extracervical extension.
In a prospective surgicopathologic evaluation of 645 patients with clinical stage IB disease who underwent radical hysterectomy and pelvic lymphadenectomy, independent prognostic factors for disease-free survival included pelvic nodal metastases, clinical tumor size (within stage IB classification), capillary-lymphatic space invasion, and relative and absolute depth of tumor infiltration into the cervical stroma.34
In more advanced lesions, a multivariate analysis of 642 patients entered into three Gynecologic Oncology Group (GOG) prospective clinical trials of definitive radiotherapy demonstrated that the presence of positive para-aortic nodes was the single most important independent predictor for relapse and survival, overwhelming all other risk factors. The next two most important prognostic factors (pelvic nodal involvement and tumor size) retained significance only in the absence of para-aortic metastases. Other weaker prognostic variables included clinical stage, patient age, and performance status. In this analysis of more advanced tumors, cell type, histologic grade, and pretreatment hematocrit and peritoneal cytologic findings were not significant prognostic factors when the preceding variables were taken into account.41
Molecular Biology
HPV is a small, double-stranded DNA virus that belongs to the papovavirus group. To date, more than 80 different strains of HPV have been isolated using polymerase chain reaction analysis.42 HPV infection occurs in the basal cell layer of the epithelium, which becomes a continuous reservoir of HPV DNA as the viral genome replicates itself in the dividing cells. As the epithelial cells mature, viral DNA replication continues in the absence of basal cell division, increasing the HPV DNA copy number per cell.43 Microscopically, this process is associated with koilocytosis, a finding considered pathognomonic of HPV infection.44
Of the many strains that have been characterized, HPV-16 and HPV-18 have been most commonly associated with squamous cell carcinoma and adenocarcinoma, respectively.45,46 HPV-31, HPV-33, and HPV-35 have also been associated with malignancy, whereas HPV-6 and HPV-11 are usually associated with benign viral condyloma or mild dysplastic epithelial changes such as CIN1.45,47–52
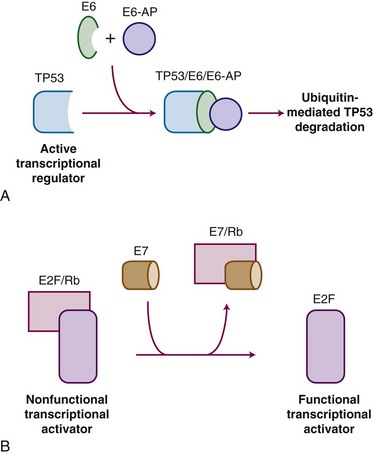
HPV carcinogenesis is mediated through oncogenes E6 and E7. Transgenic mouse experiments provide some of the strongest in vivo evidence linking E6 and E7 expression to the development of cervical cancer.53,54 Figure 56-1 outlines the mechanisms by which HPV influences oncogenic activity. The E6 protein of HPV oncogenes binds with the E6-associated protein (E6-AP) to the TP53 protein, which results in the degradation of TP53, an important negative regulator of cell growth. Thus, by binding and degrading the TP53 protein, E6 proteins contribute to the pathogenesis of cancer by removing the critical and protective function of TP53.55 The E6 proteins of oncogenes from a carcinogenic HPV strain, such as HPV-16 and HPV-18, bind TP53 more efficiently than oncogenes from HPV strains not involved in carcinogenesis, which possibly explains the differences in oncogenicity of these strains.56 By itself, E6 is not capable of inducing transformation, but it induces immortalization of primary human keratinocytes in conjunction with E7, the protein of which binds to and functionally inactivates the gene product of the retinoblastoma (RB) tumor suppressor gene. This binding results in the uncontrolled release of active transcription factors (E2F) and unregulated progression through the cell cycle. Biologic differences between low-risk and high-risk HPV viruses include observations that the latter are capable of inducing chromosome abnormalities in normal keratinocytes and are more likely to interfere with cell cycle regulatory proteins and checkpoints.57
The evidence implicating HPV in the pathogenesis of cervical cancer is conclusive. It includes (1) multiple epidemiologic studies demonstrating HPV infection as the most important risk factor for the development of squamous cell intraepithelial lesions and cervical carcinomas, (2) detection of HPV DNA in more than 90% of cervical cancers and their precursor lesions, (3) evidence of HPV transcriptional activity in neoplastic tissues, and (4) evidence that HPV oncogenes can mediate malignant transformation in transgenic mice.53,54 The precise rate of development for both low- and high-grade squamous intraepithelial lesions in women infected with HPV is unknown. Longitudinal studies have demonstrated that the incidence of HPV infections among cytologically normal sexually active young women is high.58 Most women infected with oncogenic HPV eliminate the infection and are at low or no risk for developing cervical neoplasia. The median duration of most new HPV infections is less than 1 year.58 More than one-third of women remain consistently or intermittently positive for HPV DNA, and many of these women will subsequently test positive for an HPV type that is different from the original HPV type.59 The transient nature of HPV infections in younger women substantiates the common observation that low-grade cervical neoplasia frequently regresses to normal.60
Theoretically, other cofactors may interact with HPV to attenuate the body’s immune response, promote persistent HPV infection, up-regulate E6/E7 expression, or enhance the genetic damage caused by HPV oncogene expression. Possible factors include smoking, oral contraceptive use, and infection with HIV.61 Several case-control studies have linked smoking behavior to an increased risk of cervical neoplasia.62,63 Carcinogens found in cigarette smoke are concentrated in cervical mucus,64 and women who smoke have decreased numbers of antigen-presenting cells in cervical epithelium.65 Thus smoking may increase the risk for cervical cancer via changes in local immunity. Immunosuppression has long been recognized as a risk factor for the development of cervical carcinoma.66 A positive link between oral contraceptive use and cervical cancer, however, is still uncertain.67 Although other cofactors may be involved, the acquisition and persistence of HPV infection are critical to the development of cervical neoplasia.
With this knowledge, specific vaccines could be developed. Koutsky and colleagues68 reported results from the first prospective, double-blind, placebo-controlled study of a monovalent HPV-16 virus-like vaccine. With a median duration of follow-up of 17 months, the incidence of persistent HPV-16 infection was 3.8 per 100 woman-years at risk in the placebo group versus 0 per 100 woman-years in the vaccine group.68
Subsequent, longer-term studies have confirmed the efficacy in preventing infection of HPV vaccines and their safety. A randomized, double-blind phase II study using a quadrivalent HPV (types 6, 11, 16, and 18) vaccine showed that the combined incidence of HPV infection or genital tract disease fell by 90% in those receiving prophylactic vaccine.69 Another long-term follow-up study of patients participating in a double-blind, randomized, placebo-controlled trial using a bivalent HPV (types 16 and 18) vaccine showed that, up to 4.5 years, the vaccine was highly immunogenic and safe, and induced a high degree of protection (100%) against HPV-16 or HPV-18 infection and associated cervical lesions.70 The quadrivalent and bivalent vaccines are both approved by the Food and Drug Administration (FDA) for women 9 to 26 years of age and are commercially available for intramuscular injection (three-dose sequence at 0, 2, and 6 months).
Using mathematical models, Kim and Goldie71 predicted that the cost-effectiveness of HPV vaccination programs in the United States will ultimately depend upon the duration of vaccine immunity and will be optimized by achieving high coverage in adolescent girls, targeting catch-up efforts for women up to 18 or 21 years of age, and revising screening practices. Unfortunately, research has shown that targeted approaches to HPV vaccination based on specific risk factors is a poor strategy for implementation, and, therefore, comprehensive strategies are recommended.72
Clinical Manifestations, Patient Evaluation, and Staging
Staging and Patient Evaluation
The current clinical staging system for cervical cancer by the International Federation of Gynecology and Obstetrics (FIGO) was updated in 200973,74 and is detailed in Table 56-2. The only changes made from the previous 1994 classification were the elimination of stage 0 (preinvasive disease) and the subdivision of stage IIA into IIA1 (tumor ≤4 cm in size) and IIA2 (tumor >4 cm). The subdivision of stage IIA parallels that of stage IB disease and reflects a partial appreciation of the prognostic impact of tumor size.
According to FIGO, only the results of palpation, inspection, colposcopy, endocervical curettage, hysteroscopy, cytoscopy, proctoscopy, intravenous urography, and plain films of the lungs and skeleton can be used to influence stage assignment. Suspected bladder or rectal involvement should be confirmed by biopsy. Because sophisticated imaging is unavailable for many in developing countries with a high incidence of locally advanced disease, FIGO staging clearly precludes the use of other imaging studies, such as lymphangiography; CT, MRI, or PET scanning; or the findings of operative procedures, including laparoscopy or open lymphadenectomy. These cannot be used to change the stage assignation. However, if CT is obtained, the urographic findings may be used to rule out hydronephrosis. The results of these other tests are of prognostic value and often influence treatment decisions, but institutions that publish reports on cervical cancer must adhere strictly to FIGO’s rules for staging to enable comparisons using consistent data among studies. Although FIGO continues to depend on a predominantly clinical staging approach, it recognizes other clinicopathologic risk factors, such as lymphovascular space invasion and lymph node status, and advises that these features, although not affecting staging per se, be recorded when determined. FIGO also acknowledges the potential benefits of contemporary imaging studies and surgical staging, where available, in helping guide treatment.74
Surgical staging of the pelvic and para-aortic lymph nodes has been used to obtain prognostic information, to identify patients who would benefit from extended-field irradiation, and to debulk grossly enlarged lymph nodes. Early reports of high complication rates in patients treated with radiation following transperitoneal lymphadenectomy discouraged the use of this procedure,75,76 but more recent studies have documented a significantly lower incidence of major complications if the operation is performed using a retroperitoneal approach.77 Laparoscopic biopsy of pelvic and aortic lymph nodes is less invasive, with shorter times to recuperation and less potential for adhesions, thereby decreasing the potential for treatment-related bowel toxicity.78,79 Using a variety of surgical approaches, Goff and colleagues80 reported that pretreatment surgical staging resulted in modifications in planned radiation therapy in 43% of patients. The value of pretreatment surgical debulking of tumor-involved lymph nodes is unproven, and although the information gained from surgical staging may be valuable in selected patients, its routine use should probably be limited to investigational settings.81
Other studies used to assess the extent of lymph node involvement include CT, MRI, lymphangiography, and PET. Lymphangiography has previously been used to assist the design of radiation fields; although it was determined to be a good imaging technique for assessing the status of the para-aortic lymph nodes,9 it is no longer available in most medical communities.
MRI of the pelvis is a relatively new modality that has been increasingly used to evaluate local tumor extent.82 MRI provides better definition of the extent of disease within the cervix than CT and may be valuable in detecting extension through the periphery of the cervix; it therefore may be particularly useful in assessing patients with disease confined to the cervix for whom surgery is being considered as primary treatment.83 MRI may also be useful for detecting extension into the uterine body. Pelvic MRI and CT are both useful for designing radiation portals, particularly in defining the position of the draining lymph nodes and, therefore, the borders of and shielding on conformal radiation fields.83,84 There appears to be no advantage with MRI over CT for detecting involved nodes.
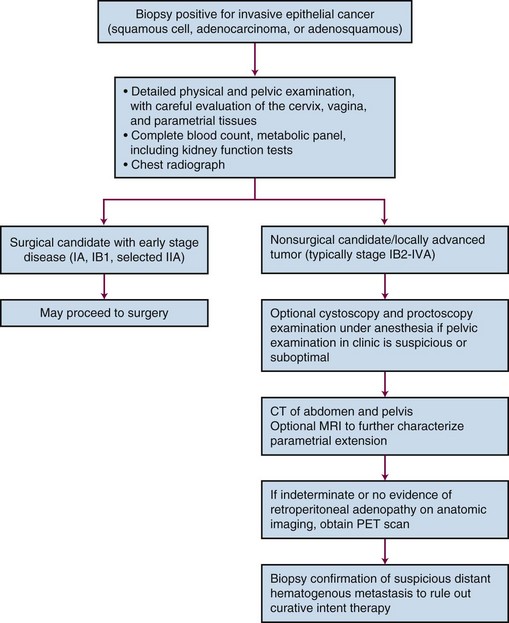
PET scanning has been compared with CT scanning for assessing lymph node status prior to planned radiation therapy. Grigsby and associates85 compared PET with CT in a retrospective study of 101 consecutive patients with newly diagnosed cervical carcinoma. CT demonstrated enlarged pelvic lymph nodes in 20% and aortic lymph nodes in 7% of patients. PET demonstrated abnormal uptake in pelvic lymph nodes in 67% and in aortic lymph nodes in 21% of patients. Surgical staging was not routinely performed to verify the accuracy of imaging findings. A multivariate analysis showed that the detection of positive aortic lymph nodes by PET imaging was the most important prognostic factor for progression-free survival. Yen and colleagues86 conducted a prospective evaluation of PET versus MRI and/or CT staging of patients with newly diagnosed (35%) or recurrent (65%) cervical cancer. Verification of lesions identified on imaging was obtained via surgical biopsy or clinical follow-up. Although its diagnostic accuracy was similar for local lesions, PET was superior to both MRI and CT in identifying metastatic lesions. As a follow-up to their previous retrospective study,87 investigators from the Mallinckrodt Institute of Radiology at Washington University have recently published a large prospective series of 560 cervical cancer patients who underwent pretreatment imaging with fluorodeoxyglucose (FDG) combined with PET. Overall, 47% of these unselected patients had lymph node involvement on FDG-PET, and the prognosis following therapy was highly correlated with positive lymph nodes as shown on PET, as well as the level of involved nodes.87 As of January 2005, the Centers for Medicare and Medicaid Services (CMS) has approved the use of PET scanning in the evaluation of newly diagnosed, locally advanced cervical cancer as an adjunct to conventional anatomic imaging. Its utility in staging early disease is limited, particularly due to the relatively low incidence of extrapelvic disease in this setting. A suggested approach in the workup of newly diagnosed cervical cancer is shown in Figure 56-2.
Of significant interest, but still requiring wider validation, is the potential use of FDG-PET imaging early (at 3 months) after completion of radiotherapy for early assessment of treatment efficacy, as well as possible identification of isolated failures amenable to aggressive salvage therapy.88
Primary Therapy
Because patients with cervical cancer usually present with disease that is clinically confined to the pelvis, locoregional disease control is the primary treatment challenge. Treatment with carefully tailored surgery or radiation therapy (RT) has produced impressive cure rates in patients with early-stage disease (Table 56-3).
TABLE 56-3 Primary Therapy and Survival by Disease Extent*
| Stage | Primary Therapy | 5-yr Overall Survival |
|---|---|---|
| IA1 | Cone biopsy; simple hysterectomy; brachytherapy | >98% |
| IA2 | Radical hysterectomy plus pelvic node dissection (PND); irradiation (RT) | ≥95% |
| IB1/limited IIA1 | Radical hysterectomy plus PND; RT | ~90% |
| IB2/larger IIA1/IIA2 | ChemoRT | 80% to 85% |
| IIB | ChemoRT | 70% to 75% |
| III | ChemoRT | ~50% |
| IVA | ChemoRT; selective exenteration | 15% to 25% |
| IVB | Chemotherapy; palliative RT | ~0% |
ChemoRT, Chemoradiation; RT, radiotherapy.
* The choice of primary therapy and the overall survival (OS) rates are related to disease extent at the time of initial diagnosis and staging evaluation.
Radical RT or chemoradiation is effective for patients with locoregional confined cervical cancer of any stage. Treatment must be carefully tailored to the patient and to the extent of disease but usually consists of a combination of external beam irradiation (EBRT) with concurrent chemotherapy and brachytherapy. Overall, historically reported 5-year OS of patients treated with RT alone are 75% to 85% for FIGO stage IB, 65% to 70% for FIGO stage II, 30% to 40% for FIGO stage III, and 10% to 15% for FIGO stage IVA disease.89–93 Within stage subsets, cure rates are strongly correlated with the size of the primary tumor and the extent of regional involvement.38,94
Radiation Treatment Variables
Chemoradiation
Initial efforts to identify a drug providing radiosensitization focused on the potential role of hydroxyurea with RT. Although several studies reported possible benefits, a recent meta-analysis has concluded that there was no evidence to support the use of hydroxyurea combined with RT in the treatment of cervical cancer.95
With data postulating that tumor hypoxia represented a significant cause of treatment failure following RT in patients with bulky cervical cancer, multi-institutional phase III trials consistently showed no improvement in outcome with the use of the first-generation nitroimadazole hypoxic-cell radiosensitizers misonidazole or pimonidazole combined with RT.96–100 On this basis, in 1996 the National Institutes of Health Consensus Statement on Cervical Cancer stated that there was “no proven benefit to combining chemotherapy with radiation” in locally advanced cervical cancer.101
Subsequently, over a relatively short time span, there was a profound shift in the paradigm of RT in the management of cervical cancer. In February 1999, the National Cancer Institute (NCI) issued a clinical announcement stating that “strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiation therapy in women who require radiation therapy for treatment of cervical cancer.”102 This recommendation was based on the results of five phase III randomized clinical trials, conducted in North America under the aegis of the NCI-sponsored Clinical Trials Cooperative Groups. The five studies had different eligibility criteria, but in total included a broad spectrum of clinical presentations: (1) patients with locally advanced tumors for whom chemoradiation represented primary therapy, (2) bulky early-stage cancers in which chemoradiation was delivered prior to adjuvant hysterectomy, and (3) postradical hysterectomy cases with high-risk pathologic factors (positive lymph nodes, positive parametria, positive margins) for whom adjuvant chemoradiation was given. There was a consistent statistically significant survival advantage favoring the RT arm that included a concurrent cisplatin-based regimen, as compared with RT alone or RT combined with hydroxyurea, with a dramatic 30% to 50% decrease in the risk of death from cervical cancer.103–111 Although now a decade old, the outcome benefit represents the single largest contemporary therapeutic gain in the management of patients with cervical cancer, especially in those with locoregionally advanced disease. Several of these studies, which initially had relatively short follow-up intervals of approximately 3 years, have been updated with significantly longer follow-up periods.108–110 These confirm that the statistically significant survival advantage of cisplatin-based chemoradiation is maintained over the long term, and they validate the 1999 NCI clinical alert. They also suggest that for future trials in locally advanced cervical cancer, estimates of PFS and OS with median follow-up intervals of 3 years represent fairly mature results.
Cisplatin was a drug of interest because it is the single most active systemic cytotoxic agent in cervical cancer, demonstrated radiosensitizing properties in vitro, had limited adverse effects on hematopoiesis, and showed promise when combined with RT in pilot studies.112–114 The relatively nonmyelosuppressive properties of cisplatin are important when considering the impact of whole-pelvis irradiation on bone marrow function.
GOG Protocol 85/SWOG 8695
From 1986 to 1990, a total of 368 evaluable patients were entered into the two-arm joint phase III randomized trial of the Gynecologic Oncology Group (GOG) and the Southwest Oncology Group (SWOG).103 Eligible cases included locally advanced squamous cell cancer, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix (FIGO stage IIB to IVA), with negative para-aortic nodes and negative intra-abdominal metastases based on surgical staging. Radiotherapy guidelines were identical in both arms. Patients with stage IIB tumors were prescribed 40.8 Gy of EBRT directed to the whole pelvis in 24 fractions (1.7 Gy per fraction), followed by low-dose-rate (LDR) brachytherapy in one or two applications, for an additional dose of 40 Gy to point A, or a cumulative point A dose of 80.8 Gy. Further limited supplemental parametrial EBRT was permitted to bring the point B dose to 55 Gy (combining both EBRT and brachytherapy contributions). Patients with stage III or IVA tumors received 51 Gy of EBRT in 30 fractions to the whole pelvis, followed by intracavitary brachytherapy delivering an additional dose of 30 Gy to point A (cumulative point A dose of 81 Gy), plus an optional parametrial boost to bring point B to a total dose of 60 Gy. Patients randomized to the control arm received hydroxyurea orally at a dose of 80 mg/kg twice weekly during EBRT. Patients in the experimental arm received cisplatin and 5-fluorouracil (5-FU) during weeks 1 and 5 of EBRT. Cisplatin was given as a slow intravenous bolus at 50 mg/m2 on days 1 and 29, and 5-FU was delivered as a 4-day infusion of 1000 mg/m2 per 24 hours on days 2 to 5 and 30 to 33 of EBRT.
With a median at-risk follow-up interval of 8.7 years, there were statistically significant improvements in disease-free survival (DFS) and OS favoring the patients treated with cisplatin and 5-FU (5-year OS of 62% in the cisplatin/5-FU arm vs. 50% in the hydroxyurea arm; p = .018) (Fig. 56-3). Analysis of patterns of first sites of relapse did not identify significant differences between the two treatment groups. The cisplatin/5-FU arm was associated with a lower incidence of severe or life-threatening leukopenia. Although there was a slight increase (not statistically significant) in acute gastrointestinal toxicity in the cisplatin/5-FU arm, the overall late complication rates at 3 years were identical for both patient cohorts.103
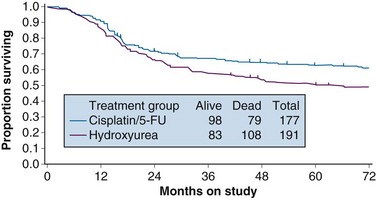
Figure 56-3 Overall survival by treatment arm in GOG 85 (p = .018).
From Whitney CS, Sause W, Bundy BN, et al: Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes. A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 17:1344, 1999, Figure 2. Reprinted with permission from the American Society of Clinical Oncology.
GOG Protocol 120
While awaiting the maturation of data from GOG 85/SWOG 8695, the GOG conducted another phase III randomized trial of concurrent chemoradiation in patients with locally advanced cervical cancer.104 Eligibility criteria for GOG 120 were similar to those in the previously described GOG 85, and included patients with locally advanced (FIGO stage IIB to IVA) cervical cancer with negative para-aortic nodal and intraperitoneal metastases following surgical staging. Radiation guidelines in this study were identical to those in GOG 85/SWOG 8695, and were held constant across all three study arms. Given that the results from GOG 85/SWOG 8695 were not yet known, the control arm was also set up as hydroxyurea given concurrently with RT, with the drug given at 3 g/m2 twice weekly during EBRT. The two experimental arms each contained cisplatin but with different schedules. In one arm, patients received cisplatin at 50 mg/m2, followed by infusional 5-FU at 1000 mg/m2/day for 4 days, starting on days 1 and 29 of EBRT (similar to GOG 85/SWOG 8695), and hydroxyurea at 2 g/m2 twice weekly throughout EBRT. In the other cisplatin treatment group, patients received cisplatin as the sole chemotherapy agent at 40 mg/m2 weekly during EBRT.
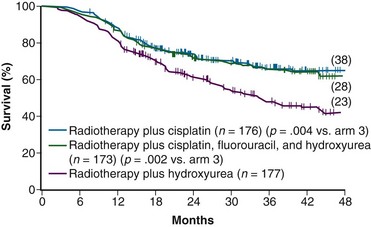
A total of 526 analyzable patients were entered into this trial from 1992 to 1997. With a median at-risk follow-up interval of 35 months, there was a significant improvement in PFS and OS favoring both cisplatin-containing arms over the arm with RT and hydroxyurea alone. Actuarial 3-year OS was 65% for both cisplatin-containing arms versus 47% for the hydroxyurea alone group (p <.005 for both cisplatin-containing arms compared with the hydroxyurea cohort) (Fig. 56-4). Patients in both cisplatin-treated groups had a significantly lower frequency of pelvic relapse (19% to 20%) compared with the patients receiving hydroxyurea alone (30%). There was also a slight reduction in distant failures favoring the cisplatin-treated patients, but this did not reach statistical significance. Although both cisplatin-containing arms had essentially identical PFS and OS, treatment with cisplatin alone resulted in significantly less acute toxicity than with the three-drug regimen, and hence it was selected as the preferred regimen from this study.104
A published update of this study, with a median at-risk follow-up interval of 106 months, confirmed prolonged improved PFS and OS favoring the cisplatin-containing chemoradiation arms, with the survival benefit seen for both stage IIB and IIIB presentations.109 Within the limitations of the available follow-up data, there was no observed increase in late toxicity with cisplatin-based chemoradiation.
RTOG Protocol 90-01
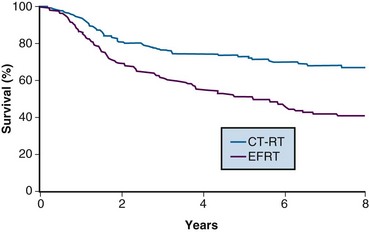
One of the many studies performed is of particular importance because it influences understanding of potential control of clinically occult para-aortic nodal disease. From 1990 to 1997, the Radiation Therapy Oncology Group (RTOG) conducted a phase III randomized trial (RTOG 90-01) comparing RT alone with concurrent chemoradiation105,108 (Fig. 56-5). Eligible patients included women with FIGO stage IIB to IVA locally advanced epithelial cervical cancers, as well as those with earlier-stage IB to IIA tumors if the primary lesion size was 5 cm or larger or if there were biopsy-proved pelvic nodal metastases. Patients with known extrapelvic disease, as evaluated by bipedal lymphangiography or retroperitoneal surgical staging, were ineligible. Based on an earlier RTOG study that indicated a benefit of prophylactic para-aortic nodal RT in patients with stage IB2 to IIB cancers,115 the control arm of RT alone included contiguous extended-field coverage to the L1 to L2 vertebral interspace. The pelvis and para-aortic nodes were prescribed a dose of 45 Gy in 25 fractions, supplemented by LDR intracavitary brachytherapy to boost the point A cumulative dose to at least 85 Gy. Institutions were allowed to initiate brachytherapy earlier, at an EBRT dose of 20 to 30 Gy, provided that the final point A dose specification was adhered to and that further pelvic EBRT be delivered with a midline block. In the experimental arm, patients were selected to receive pelvic RT only, with the superior field edge defined at the L4 to L5 vertebral interspace. With the exception of eliminating prophylactic para-aortic coverage, EBRT parameters for the experimental arm were similar to those of the control group. Concurrent with RT, patients randomized to the experimental group received three cycles of cisplatin/5-FU chemotherapy (cisplatin 75 mg/m2, 5-FU 1000 mg/m2/day for 4 days) administered at 3-week intervals, with the third cycle often given at the time of brachytherapy insertion.105,108
A total of 388 analyzable patients were included. At a median at-risk follow-up interval of 43 months, preceding the NCI clinical announcement, there was a statistically significant advantage for DFS and OS favoring the chemoradiation arm.105 Actuarial 5-year OS was 73% in patients treated with chemoradiation compared with 58% in patients treated with extended-field RT alone (p = .004).
The initial results of RTOG 90-01 were updated in the 388 analyzable patients with a median at-risk follow-up duration of 6.6 years.108 The significant outcome benefit of concurrent chemoradiation over RT alone has been maintained, with an 8-year OS of 67% versus 41%, respectively (p <.0001) (see Fig. 56-5). In a post hoc analysis, the advantage of chemoradiation was noted regardless of FIGO stage (IB and II vs. III and IVA), pelvic nodal involvement, or the method of pretherapy nodal evaluation (lymphangiography vs. retroperitoneal nodal staging). The rate of cumulative overall grade 3 or higher late complications was similar in both treatment groups (14% at 5 years). Analysis of patterns of failure noted a significant reduction in locoregional and distant metastasis (excluding the para-aortic nodes) relapse rates favoring the patients treated with RT and concurrent cisplatin/5-FU. Although there was a slightly higher para-aortic failure rate in patients receiving chemoradiation compared with extended-field RT alone, this did not reach statistical significance.
The patterns of failure recorded in RTOG 90-01 (Table 56-3) may allow the hypothesis that concurrent chemotherapy not only provides locoregional radiosensitization but also addresses preexisting micrometastases, although it should be recognized that the risk of distant metastases is closely linked to the incidence of pelvic failure, which was higher in the control arm.39 At the very least, this trial indicates that for patients at some but variable risk of para-aortic nodal metastases, albeit without grossly identifiable disease, chemoradiation with irradiation limited only to the pelvis achieves better outcomes than extended-field irradiation alone without chemotherapy.
GOG Protocol 123
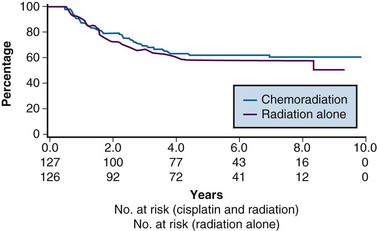
From 1992 to 1997, the GOG conducted a phase III randomized trial comparing preoperative treatment with RT versus RT and concurrent cisplatin in patients presenting with an earlier tumor stage than those included in the preceding three described studies.106 Eligible patients were those with stage IB2 cervical cancers (≥4 cm tumor diameter), with no evidence of retroperitoneal adenopathy on radiologic (CT or lymphangiography) or surgical assessment. Radiotherapy specifications were identical in both treatment arms. The pelvis was prescribed a dose of 45 Gy in 25 fractions with EBRT, followed by LDR intracavitary brachytherapy to boost the cumulative point A dose to 75 Gy. In the experimental arm, patients received weekly cisplatin at a dose of 40 mg/m2 (with a maximal dose of 70 mg/week) for up to six doses. The final dose of cisplatin could be given during brachytherapy insertion. Based on the interim results of a previous GOG trial that evaluated the role of adjuvant hysterectomy in centrally bulky tumors (GOG 71), all patients in this study underwent extrafascial hysterectomy following either RT alone or RT plus concurrent cisplatin.
A total of 369 patients were evaluated, with a median at-risk follow-up interval of 36 months. Patients who received concurrent cisplatin had a significantly higher incidence of pathologic complete response in the hysterectomy specimen, as well as improved rates of pelvic control, PFS, and OS compared with those treated without chemotherapy. Three-year OS was 83% and 74% in the chemoradiation versus RT arms, respectively (p = .008)106 (Fig. 56-6). Although there was an increase in acute toxicities in the patients treated with cisplatin and radiation, these reactions were predominantly of transient hematologic perturbations, and severe late toxicities were infrequent and equally divided between the two groups. An updated analysis of this study, with an extended at-risk follow-up duration of 101 months, confirmed that concurrent weekly cisplatin with RT maintained significant long-term PFS and OS benefit compared with RT alone, without a reported increase in serious late effects.110
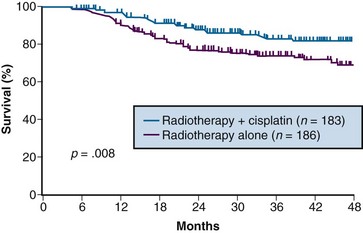
Figure 56-6 Overall survival by treatment arm in GOG 123 (p = .008).
From Keys HW, Bundy BN, Stehman FB, et al: A comparison of weekly cisplatin during radiation therapy versus irradiation alone, each followed by adjuvant hysterectomy in bulky stage IB cervical carcinoma. A randomized trial of the Gynecologic Oncology Group. N Engl J Med 340:1154-1161, 1999. Copyright © Massachusetts Medical Society. All rights reserved.
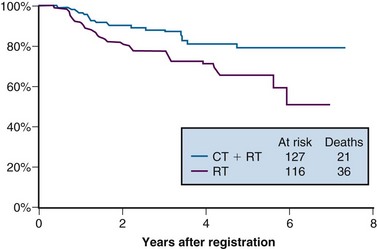
Intergroup 0107 (SWOG 8797/GOG 109/RTOG 91-12)
The last of the five phase III trials that formed the basis for the 1999 National Institutes of Health (NIH) clinical announcement investigated the role of chemoradiation as an adjunct to primary surgery for patients with clinical early-stage disease in whom postoperative high-risk pathologic features had been discovered.107 Patients eligible for this intergroup study were those with stage IA2, IB, or IIA carcinoma of the cervix who were initially treated with radical hysterectomy and pelvic lymphadenectomy and who were found to have positive pelvic lymph nodes, positive margins, and/or positive parametrial infiltration on microscopic evaluation. Taken together, these high-risk factors have the unifying theme of pathologically identified extracervical/extrauterine disease extension. In this two-arm trial, patients were randomized to pelvic RT alone (EBRT to 49.3 Gy in 29 fractions, without brachytherapy) or to the same irradiation combined with chemotherapy. Chemotherapy consisted of cisplatin (70 mg/m2) and 5-FU (1000 mg/m2/day for 4 days) given for four cycles beginning on days 1, 22, 43, and 64 of therapy. Two of the chemotherapy cycles were delivered concurrently with pelvic RT, followed by two additional cycles after completion of EBRT.
A total of 243 eligible patients were entered into the trial from 1991 to 1996. With a median at-risk follow-up time of 42 months, patients receiving combined adjuvant chemoradiation had statistically significant improvement in PFS and OS compared with those receiving RT alone. Estimated 4-year OS was 81% versus 71% for the chemoradiation and RT only arms, respectively (p = .007) (Fig. 56-7). The outcome benefit for adjuvant chemoradiation appeared to arise from reduction in both pelvic and distant failures, although comparisons of relapse patterns between treatment arms did not reach statistical significance. There was an increase in acute toxicities in the combined-modality arm, but these were mostly limited to transient and manageable hematologic and gastrointestinal effects.111
National Cancer Institute of Canada Trial
In contrast to the consistent therapeutic benefits seen for combining cisplatin-based chemoradiation in the preceding five phase III trials, the National Cancer Institute of Canada (NCIC) published the results of another randomized study that questioned the role of concurrent chemoradiation in locally advanced cervical cancers.111 Eligible patients included those with stage IIB to IVA squamous cell cancers or earlier-stage disease (IB to IIA) if the primary tumor was at least 5 cm in diameter or if there was histologically positive pelvic nodal involvement. Radiation specifications, which were identical in both treatment arms, prescribed a dose of 45 Gy in 25 fractions to the pelvis with EBRT. This was followed by intracavitary brachytherapy using various allowable dose rates, but each calibrated to provide an LDR biologically equivalent dose of 35 Gy to point A (total cumulative point A dose of 80 Gy in conventional equivalents). Careful quality control was maintained to complete all RT within 7 weeks. Patients randomized to receive chemotherapy were given weekly cisplatin at a dose of 40 mg/m2 for a total of five administrations concurrently with EBRT.
Between 1991 and 1996, 253 eligible patients were entered into the trial. With a median at-risk follow-up of 82 months, there were no observable differences in PFS or OS in the two treatment arms (Fig. 56-8).
Various reasons for the lack of a demonstrable effect of cisplatin concurrent with RT in the NCIC study have been promulgated by the authors and others. These include the lack of surgical staging, a relatively small sample size, the confounding factor of treatment-related anemia in the chemotherapy arm, and the omission of 5-FU (which may be synergistic with cisplatin) from the chemotherapy regimen. Perhaps most provocative is the suggestion that concurrent cisplatin-containing chemoradiation exhibits its greatest benefit primarily in patients with suboptimal and prolonged overall RT durations. In contrast to the studies reported by Whitney103 and Rose,104,109 where the median duration of the treatment course was 64 and 62 days, respectively, the timing for completion of RT in the NCIC study was carefully controlled, with a median duration of 51 days.111 The potential reduced impact of concurrent cisplatin with optimum RT schedules has been refuted by others, however.116 Nonetheless, the NCIC authors conclude that despite their negative trial, “the balance of evidence favors the use of combined-modality treatment for the types of patients studied in this trial. The best results are certainly achieved by careful attention to RT details, including dose and overall delivery time, the use of brachytherapy whenever possible, and probably the addition of concurrent cisplatin chemotherapy to RT.”111
Chemotherapy Concurrent with Irradiation: A Synopsis
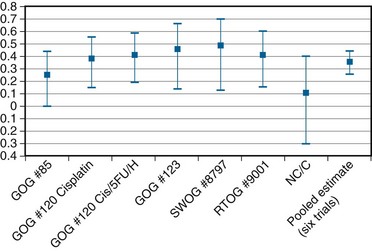
In an overview, Rose and Bundy116 summated the collective results of the six North American randomized trials (including the NCIC study) and showed a cumulative and statistically significant 36% reduction in the risk of death favoring combined cisplatin-based chemoradiation over RT alone or combined with hydroxyurea116 (Fig. 56-9). The generalizability of concurrent chemoradiation for cervical cancer to an unselected, non–study-limited patient population has also been raised. In part, this has been addressed by a provocative population-based cohort study of cervical cancer outcome in Ontario, Canada. In this analysis, Pearcey and colleagues117 showed a significant improvement in cervical cancer OS at the population level between 1992 and 2001, without a coincident change in stage presentation or an imbalance in cases selected for primary surgery. The investigators attribute the survival gains to the generalized and appropriate use of concurrent, predominantly cisplatin-based chemoradiation in the majority of patients receiving RT for cervical cancer.117
Although cisplatin-based chemoradiation has formed the basis for most discussions and conclusions regarding the superiority of combined-modality therapy, more recent data suggest that other nonplatinum agents, most notably 5-FU and/or mitomycin C, may also provide benefit when used concurrently with definitive radiotherapy. A recent updated and comprehensive meta-analysis of chemoradiation for locally advanced cervical cancer confirmed the general applicability of this treatment algorithm for all women and endorsed the NCI alert regarding the use of concomitant cisplatin-based chemoradiotherapy but also indicated that similar gains could be derived from non–platinum-based chemotherapy.118
It should be emphasized, however, that RT remains the single most effective modality in the management of patients with locally advanced cervical cancer, where surgical extirpation of tumor is infeasible or incomplete. Even in the absence of chemotherapy, RT alone is able to cure many patients with bulky cervical tumors. Some patients present with hydronephrosis and renal dysfunction or other medical comorbidities that preclude the use of cisplatin. The use of concurrent carboplatin, which avoids nephrotoxicity, has been suggested by some as an alternative to cisplatin, but its comparative efficacy remains unproven.119 Ultimately, the selection of concurrent cisplatin-based chemotherapy requires sound medical judgment, balancing the potential improvement in tumor control with patient physiology, disease presentation, and the increased acute toxicities (notably, hematologic, metabolic, and gastrointestinal) associated with the addition of chemotherapy.
Noncisplatin Chemoradiation Regimens
Although there is general acknowledgment of the efficacy of cisplatin in combination with RT, various investigators have formally evaluated other noncisplatin chemoradiation regimens, primarily infusion 5-FU. The GOG conducted a phase III trial (GOG 165) in which patients with locally advanced stage IIB to IVA tumors were randomized to weekly cisplatin at 40 mg/m2/week versus protracted venous infusion of 5-FU at 225 mg/m2/day for 5 days per week throughout the duration of EBRT. GOG 165 was closed before planned completion when a scheduled interim analysis indicated that there was no likelihood that the 5-FU arm would ever show improved outcomes compared with the control arm of weekly cisplatin.120 Other concurrent chemoradiation regimens tested that appear to have potential benefit include epirubicin121 and 5-FU plus mitomycin C.122
Balanced against the indeterminate individual trial results described above, a recent comprehensive meta-analysis has suggested that nonplatinum agents may also improve outcome when combined with RT and deserve further evaluation.118
Neoadjuvant Chemotherapy
Although concurrent cisplatin-based chemoradiation has become the widely accepted standard of care, caution is raised regarding the use of strategies employing neoadjuvant chemotherapy before RT. Multiple randomized trials, most with small patient numbers, have failed to identify an effective strategy of neoadjuvant chemotherapy followed by definitive RT. A systematic review and individual patient data meta-analysis showed no benefit and, indeed, an overall trend to detriment, for patients receiving neoadjuvant chemotherapy (predominantly cisplatin-based) before radical RT.123 In two of the randomized trials, it was reported that the neoadjuvant chemotherapy approach, although associated with a reasonable initial response, ultimately resulted in statistically significant inferior pelvic control and OS compared with definitive RT alone.124,125
A plausible explanation for the lack of benefit seen with chemotherapy preceding RT is that although responses can be seen with systemic cytotoxics alone, the remaining clonogens undergo accelerated repopulation, thereby offsetting the efficacy of RT.126 Questions remain regarding the potential benefit of initial chemotherapy in surgical candidates or where the dose and intercycle interval of systemic agents are optimized, but the use of neoadjuvant chemotherapy strategies should be considered only in the context of carefully designed clinical trials. Unless more effective cytotoxic agents are identified, there is no current reason to consider neoadjuvant chemotherapy followed by definitive RT or chemoradiation in patients with localized, curable cervical cancer.
Brachytherapy Plus External Beam Irradiation
The importance of brachytherapy in the curative treatment of intact cervical cancer is indisputable. Clinicians have sometimes been inclined to deemphasize the use of brachytherapy in patients with FIGO stage III disease, arguing that disease at the pelvic wall is beyond the reach of traditional intracavitary therapy. Reports from the Patterns of Care Study127 and from the M.D. Anderson Cancer Center (MDACC),94 however, have demonstrated significantly better survival rates for patients treated with a combination of EBRT and intracavitary irradiation. In the MDACC report of 1007 patients treated with irradiation for FIGO stage IIIB disease, the disease-specific survival (DSS) was 43% for patients who had intracavitary irradiation versus 21% for those treated with EBRT alone. Patients treated during periods when policies emphasized the use of brachytherapy had significantly better survival rates. Patients who received higher doses of EBRT (and concomitantly lower doses of intracavitary irradiation) to the central pelvis had poorer survival rates. The rate of major complications was also correlated with the proportion of central pelvic treatment given with EBRT, and this correlation suggested that the therapeutic ratio narrows when the dose of EBRT given to the whole pelvis exceeds 45 Gy.94 Nonetheless, a careful balance between EBRT and brachytherapy components of therapy, accounting for tumor volume, anatomic presentation, and disease regression rate, represents the hallmark of appropriate cervical cancer radiotherapy planning.
Radiation Dose and Duration
The adverse impact of treatment duration protraction in cervical cancer has been evaluated by several investigators. There is general consensus that prolonging a course of radical RT beyond approximately 7 weeks is associated with a 0.5% to 1% decrease in pelvic control per extra day.128–133 Although debate remains about whether an increase in treatment duration adversely affects outcome or is simply a reflection of unfavorable tumor or patient characteristics, there is compelling radiobiologic demonstration of accelerated tumor repopulation that mitigates the efficacy of RT.126 In contrast, there is no evidence that prolongation of treatment duration results in a reduction of late normal tissue sequalae.133 Therefore it is now considered prudent to complete a course of definitive RT for cervical cancer within 7 to 8 weeks by eliminating all elective treatment breaks, delivering EBRT parametrial boosts between, or interdigitated with, brachytherapy insertions, and actively supporting patients through acute toxicities so as to avoid RT interruptions.
Role of Extended-Field Irradiation
The prognosis of patients with cervical carcinoma is inversely related to the extent of regional and nodal involvement. Because hematogenous metastasis is usually a late event in the course of this disease, however, patients with lymph node metastases often can be cured with regional EBRT if pelvic disease can be controlled. Even patients with documented para-aortic lymph node metastases have 5-year OS of 20% to 50%, depending on the extent of pelvic disease.134–139
Two groups have explored the use of prophylactic extended-field EBRT in patients who do not have overt para-aortic metastases. A trial from the European Oncology and Radiation Therapy Consortium (EORTC)140 was conducted prior to the use of concurrent chemotherapy. Randomized patients had more locally advanced pelvic disease (bulky FIGO stage IIB, FIGO stage III, or biopsy-proved pelvic lymph node metastases). DFS of patients in the two treatment arms was not significantly different at 4 years, although the rate of relapse in the para-aortic lymph nodes was higher in patients who had not received RT to this site. OS was not reported. Results from RTOG 90-01 were previously discussed.
Although the role of prophylactic para-aortic nodal RT remains to be fully defined, the results of RTOG 90-01 clearly indicate that concurrent chemotherapy with pelvic RT leads to significant outcome benefits over extended-field EBRT without chemotherapy.108 Underlying the question of benefits of prophylactic para-aortic RT is the assumption that the para-aortic nodes may represent isolated occult extrapelvic disease in some patients for whom sterilization by RT would translate to cure (assuming achievable pelvic control and no distant metastases). The results of RTOG 90-01, which showed significant reductions in both pelvic and distant relapse with chemoradiation, suggest that the use of concurrent chemotherapy to some degree addresses subclinical metastases beyond the volume of RT. Unanswered is the question of whether prophylactic extended-field RT in addition to systemic chemotherapy would provide additional therapeutic gain.
Practice patterns vary for patients with negative para-aortic nodes but who are deemed to be at elevated risk for occult disease in this nodal chain. At the University of Washington, prophylactic para-aortic nodal EBRT to 45 Gy, concurrent with chemotherapy (to the level of the renal vessels, or at the L1 to L2 vertebral interspace), is often considered for patients with the highest potential risk of subclinical para-aortic disease (i.e., those with common iliac adenopathy on surgical staging or those who have negative para-aortic nodes but extensive pelvic lymphadenopathy by radiologic imaging [recognizing the limitations of even the best imaging modalities to detect microscopic tumor]). Whereas the efficacy of such an extended-field prophylaxis approach has not been proven, especially in the era of chemoradiation, the use of concurrent cisplatin-based chemotherapy with extended-field RT has been shown to be tolerable in several phase II trials.138,139
Impact of Anemia and Tumor Hypoxia on Radiation Therapy Outcome: Potential as Therapeutic Targets
The adverse association of anemia on outcome following primary RT for cervical cancer has been well documented in many previous clinical reviews.140–142 Whether this association reflects cause and effect or whether anemia is simply a surrogate for worse prognostic and predictive variables, such as larger and more radiation-refractory tumors, is still being debated. Potential mechanisms for a negative impact of anemia on cervical cancer RT outcomes may be linked to tumor hypoxia and consequent radioresistance, as well as induction of angiogenesis, increased tumor aggressiveness, and enhanced metastatic potential.
Some retrospective reports have suggested that anemia is an independent negative prognostic factor, with the average hemoglobin level during RT being more predictive than the level before therapy.143,144 Only one small prospective randomized trial directly addressing the value of transfusion has been completed, in the 1970s. This study indicated improved pelvic control for irradiated patients whose hemoglobin level was corrected by transfusion, but the analysis was hampered by limited patient numbers and lack of stratification.145
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree