Fig. 3.1
The diagnostic algorithm for a thyroid nodule
Initial Approach to the Patient Suspected of Having a Nodule
The discovery of a thyroid nodule over 1 cm should prompt measurement of the serum TSH [7]. In a patient with a high or normal TSH, the likelihood of identifying a hyperfunctioning nodule is very low. The great majority of nodules in such patients will be either cold or warm on scanning; further evaluation of the need for FNA will be based solely on the sonographic features. As such, in patients with a normal or high TSH, scintigraphy is unnecessary (see Fig. 3.1) [7]. Conversely, a patient with a low TSH and one or more nodules requires scintigraphy to determine if the nodule(s) is(are) toxic and may be observed, or if further interrogation by fine needle aspiration is required in the event of a warm or cold nodule [7]. Consideration to scintigraphy may also be given in a patient with a cytologic diagnosis of follicular neoplasm, particularly if the serum TSH is in the low-normal range [11]. Molecular testing of such indeterminate nodules has largely replaced this approach in clinical practice, however [12].
Most benign and nearly all malignant nodules concentrate radioiodine less avidly than the surrounding normal thyroid tissue. Approximately 5 % of thyroid cancers, however, will concentrate pertechnetate but not radioiodine [13]. Such nodules may appear warm or hot on pertechnetate scans [14]. Consequently, it is recommended that patients with functioning nodules on 99mTc-pertechnetate scans should undergo confirmatory imaging with radioiodine to determine their functional status [15].
Clinician-Performed Ultrasonography
Ultrasonography is rapidly gaining popularity among non-radiologists for use in the clinical setting. In point of fact, use of ultrasonography at the bedside has become a mandatory component of training for some surgery residencies and all endocrinology fellowships. The high prevalence of thyroid nodules, particularly the nonpalpable variety, necessitates a simple stratification tool to distinguish the benign lesions from those requiring further evaluation. As such, ultrasonography has become an extension of the physical examination in the evaluation of the patient with a thyroid nodule. Indeed, a properly trained clinician can provide a similarly informative exam as that of a radiologist [16].
The benefits of an ultrasound performed by a non-radiologist are manifold. Long-recognized as a more powerful tool when simultaneously performed and interpreted by the same individual, US exams increasingly are being done instead by technologists with subsequent interpretation of the images by a radiologist. Subtle findings on US such as comet-tail signs or microcalcifications easily can be missed when only static images are reviewed. Real-time sonography, in contrast, reveals a wealth of information about the subtle morphologic characteristics of a lesion and the background thyroid parenchyma. Further, the ability to visualize a structure in multiple planes offers a unique radiologic opportunity to create a three-dimensional image, providing important details regarding geographic relationships with nearby anatomic structures. These clues can provide additional context regarding the risk of malignancy or benignity for any given nodule.
From the patient’s perspective, clinician-performed exams may be preferable; the endocrinologist or surgeon can provide immediate feedback to the patient regarding the exam findings, rather than waiting for the radiologist’s report. These results can then prompt an immediate action, such as an FNA or counseling regarding the need for surgery, in the case of a suspicious nodule or lymph node. In fact, a study of 223 patients referred to a multidisciplinary thyroid nodule clinic revealed that the use of ultrasonography in the clinic altered the management in 63 % of patients [6].
For patients with a diagnosis of thyroid cancer, performing the appropriate initial surgery is a critical determinant of outcome, to minimize the risk of residual disease [7]. Thyroid cancer has a high predilection for metastatic spread to the locoregional lymph nodes; macroscopic nodal disease may be seen in up to 50 % of patients [17–19]. Preoperative US of the neck for identification of suspicious cervical lymph nodes is strongly recommended for all patients undergoing thyroidectomy for a malignant or suspicious cytologic diagnosis [7]. An appropriate initial surgery reduces the morbidity associated with multiple operations, which are often required when the diagnosis of nodal metastases is made after the thyroidectomy [20]. Additionally, the identification of nodal metastases alters the staging and, ultimately, may result in a modification of the treatment strategy [19]. When performed by a radiologist, the presence (or absence) of nodal metastases often is not assessed on preoperative ultrasound, unless specifically requested by the ordering physician. Indeed, one study found that the preoperative US performed in a radiology center missed 88 and 93 % of patients with suspicious central and lateral lymph nodes requiring FNA compared to the findings of an US exam by a surgeon [21]. Moreover, the findings on clinician-performed exam changed the surgical approach in 45 % of patients compared to the initial assessment based on the radiologic diagnoses [21].
Performance of the Ultrasound Exam
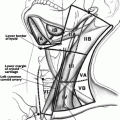
Although sonography is a relatively simple procedure to perform, there are a few essential components of the exam that should be reviewed. It is critical to place the patient in the supine position with the neck hyperextended. Such positioning allows structures in the inferior neck and superior mediastinum to be more readily visualized. Attempts to identify the inferior parathyroid glands, a substernal goiter, or mediastinal lymph nodes may be unsuccessful if this important detail is overlooked. Occasionally, it may be necessary to place a pillow or towel roll between the patient’s shoulders to achieve greater extension of the neck.
The optimal method for performance of the sonographic exam has not been defined; it is of utmost importance though to cover all areas of the neck. Additionally, the inspection should include both the transverse and longitudinal (sagittal) views. When evaluating a patient with a thyroid nodule, assessment of the lymph nodes, particularly in the lateral neck, provides important clues regarding the malignancy risk. Identification of a suspicious lymph node increases the likelihood of cancer and may warrant FNA of the node in lieu of the thyroid [7] (see Fig. 3.2 normal neck anatomy).
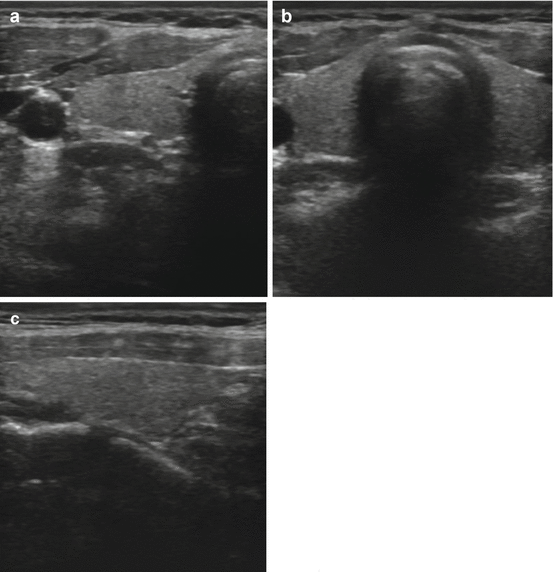

Fig. 3.2
(a) Transverse view of a normal right lobe of the thyroid. (b) Transverse view normal isthmus and left lobe. (c) Longitudinal view normal thyroid
Sonographic Risk Stratification
Ultrasonography is a highly sensitive tool for the identification of thyroid nodules; screening for such lesions identifies nodules in up to 40 % of patients [22], with increasing incidence with advanced age [1]. The high prevalence of nodules and the relative infrequency of malignancy mandate a method of stratification, as there are insufficient resources to perform an FNA of every thyroid lesion identified. Various individual sonographic features have a high specificity for identification of malignancy [23]; the absence of one of these features, however, does not rule out the possibility of a cancerous nodule.
Nodule Size, Composition, and Number
Nodule size does not correlate with malignancy risk, though nearly all consensus guidelines stratify nodules for FNA based on their largest dimension. This size threshold is a necessity, however, as it is increasingly recognized that microcarcinomas are highly prevalent and represent an indolent, clinically inconsequential tumor in the vast majority of cases [24]. The various size thresholds for FNA (see Table 3.1) have been chosen because clinically significant tumors may exist in nodules above this limit [7]. Smaller nodules, even when harboring suspicious US features, may be followed with serial US exams for growth; such a change could potentially indicate a more aggressive lesion which would then warrant interrogation with FNA.
Table 3.1
Summary of 2015 American Thyroid Association recommendations for fine needle aspiration biopsy of thyroid nodules
Nodule features on ultrasound | Recommended FNA based on nodule size |
|---|---|
High and intermediate suspicion for malignancy | ≥1 cm |
Low suspicion for malignancy | ≥1.5 cm |
Very low suspicion for malignancy (completely cystic/simple cyst) | ≥2 cm vs. observation without FNA is also an option |
Benign features (cystic) | No FNA unless symptomatic or cosmetic reasons |
Echogenicity
Thyroid parenchyma has a medium gray echotexture compared to the surrounding structures in the neck. A lesion that is of the same echogenicity as normal thyroid tissue is termed isoechoic. Those nodules which have a brighter appearance than that of the normal thyroid are labeled hyperechoic. Nodules that are either iso- or hyperechoic have a lower likelihood of malignancy. Most nodules are hypoechoic or darker than the normal thyroid parenchyma. Hypoechogenicity of a nodule is associated with a higher risk of malignancy. Indeed, most malignant nodules are hypoechoic; the majority of hypoechoic nodules, however, are not cancerous. A nodule with an echotexture that is darker than the surrounding strap muscles is termed markedly hypoechoic. Such a finding has a very high specificity for malignancy [23]. A nodule that is completely black is designated anechoic; these lesions are typically simple cysts (see Fig. 3.3).
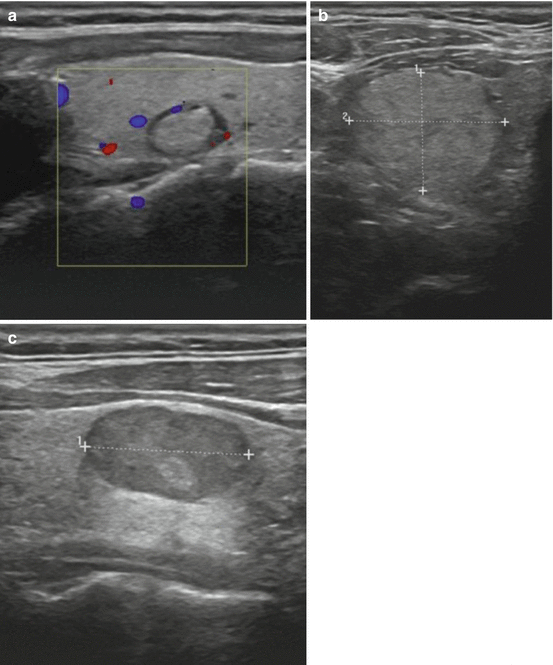
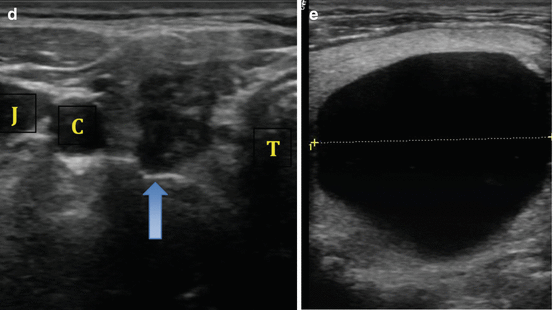


Fig. 3.3
(a) Isoechoic nodule. (b) Hyperechoic nodule. (c) Hypoechoic nodule. (d) Markedly hypoechoic nodule with lobulated borders. (e) Anechoic nodule (simple cyst)
Nodule Shape
The natural growth plane of a benign nodule is horizontal in a supine patient (or the nodule width). Growth opposite of this plane is suggestive of an aggressive lesion (see Fig. 3.4). Nodule height that is greater than the nodule width has been reported to have a specificity of 91 % for identification of malignancy, though the sensitivity for this feature is significantly lower [26].
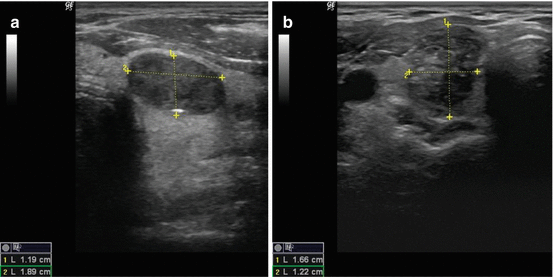

Fig. 3.4
(a) Nodule width greater than height. (b) Nodule height greater than width
Nodule margins are an important feature to assess for malignancy risk. The interface between the thyroid nodule and the surrounding normal parenchyma may be well demarcated (Fig. 3.4a) or poorly defined. It is important to note that a poorly defined margin does not necessarily translate to increased risk of malignancy and is not the same as an irregular margin. Isoechoic or mildly hypoechoic nodules are more likely to have a poorly defined border and carry a low risk of malignancy (Fig. 3.3b). In contrast, nodules that have a microlobulated, spiculated, or infiltrative margin have a high malignancy risk [26] (see Fig. 3.5).
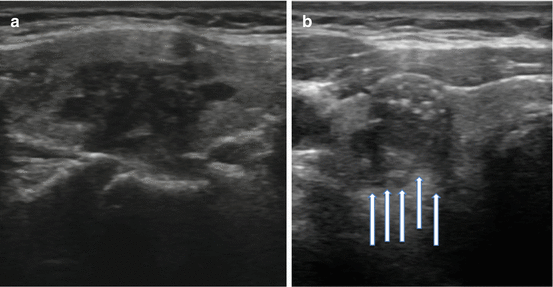

Fig. 3.5
(a) Microlobulated margin. (b) Infiltrative margin
Calcifications
Calcifications are identified on sonography as bright echogenic foci (hyperlucencies). Typically divided into one of three categories, microcalcifications, macrocalcifications (or coarse calcifications), and eggshell (linear), the risk of malignancy varies with each type (see Fig. 3.6a). Microcalcifications are associated with the highest risk of malignancy, though the presence of any of the three increases the risk in any given nodule.
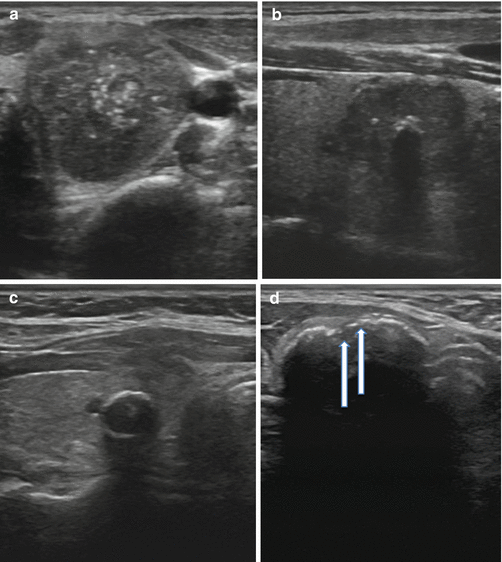

Fig. 3.6
(a) Microcalcifications. (b) Coarse calcification. (c) Continuous eggshell calcification. (d) Interrupted eggshell calcification
Microcalcifications are defined as punctate hyperlucencies (1 mm or less in size) and lack signal dropout posteriorly (see Fig. 3.6a). Macrocalcifications, in contrast, are greater than 1 mm in dimension and are associated with signal dropout posteriorly. The loss of signal results from the failure of the US wave to penetrate the calcification; consequently, the entire sound wave is sent back to the probe (see Fig. 3.6b). Such coarse calcifications within a nodule are not uniformly associated with malignancy [27]. The coexistence of coarse calcifications and microcalcifications within a single nodule confers the same risk as microcalcifications alone [28, 29]. Eggshell calcifications may be described as intact or interrupted. The interrupted variety is associated with a greater malignancy risk [30, 31].
Vascularity
Early studies have suggested that the pattern of blood flow within a nodule can be used as an additional tool to identify suspicious nodules [29, 32, 33]. More recent reviews of the topic have shown that intranodular vascularity is not associated with malignancy risk on multivariate logistic regression analysis [34].
Benign US Findings
Not all US features are associated with malignancy; various findings on sonography may be strongly associated with benignity. Among the more common reassuring findings are spongiform nodules. These nodules have a “honeycomb” appearance and are composed of multiple tiny cystic structures divided by thin septations involving more than 50 % of the nodule (see Fig. 3.7a).
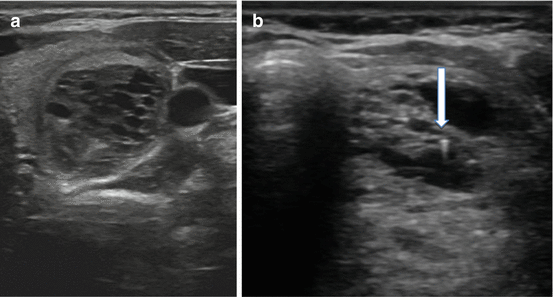

Fig. 3.7
(a) Spongiform nodule. (b) Comet-tail sign
Colloid, a normal component of the thyroid follicle and the locale for the formation of thyroid hormone, may be identified on sonography. Insipissated colloid may be seen as a comet-tail sign (ring-down artifact or cat’s eye). The energy of the ultrasound wave as it strikes the colloid crystals creates a vibration which influences their return to the transducer after the initial reflected signal. The result is a hyperlucency with a posterior tail or stepladder artifact (see Fig. 3.7b). The comet-tail sign, when found within a cystic area, is a reassuring finding most commonly associated with colloid nodules and resolving hematomas. Although rare, it may be seen in PTC, but is typically found within a solid component of the lesion [35].
Pattern Recognition
Quantification of the risk of malignancy in a given nodule based on its individual ultrasound features can be challenging, even in the hands of an experienced sonographer. Indeed, the identification of a single US parameter cannot satisfactorily identify the entire subset of patients who require FNA, as the requisite sensitivity and specificity cannot be achieved with any one sonographic feature. Additionally, the interobserver variability of individual US characteristics is unacceptably low. Instead, the majority of thyroid nodules should be classified into one of several US patterns; such a classification system has been reported to have a high interobserver correlation [36, 37]. Borrowing terminology from breast cancer imaging, various authors have proposed a thyroid imaging reporting and data system (TIRADS) for categorization of thyroid nodules based on their sonographic appearance [36, 38]. Additionally, the American Thyroid Association has created a system of pattern recognition for stratification of thyroid nodules into various risk categories [7]. This system assigns a risk of malignancy and provides a recommendation regarding the size threshold for FNA and is explained below (See Table 3.1 and Chap. 4, “Thyroid Nodule Biopsy”).
High Suspicion Nodules
A solid hypoechoic nodule or a cystic nodule with a solid component that is hypoechoic and has at least one of the following features, irregular margins (infiltrative or microlobulated), microcalcifications, taller-than-wide shape, interrupted rim calcifications, or evidence of extrathyroidal extension, is considered to be high risk nodules [7]. This group of lesions has been found to carry an estimated risk of malignancy of 70–90 % [7]. As such, it is recommended that nodules in this category should undergo FNA when they are 1 cm or larger [7].
Intermediate Suspicion Nodules
Hypoechoic solid nodules with smooth margins and lacking microcalcifications, extrathyroidal extension, or taller-than-wide shape are of intermediate suspicion [7]. This sonographic pattern has the highest sensitivity for identification of PTC at 60–80 % but carries a lower specificity than in the high risk group [7]. For this reason, it is also recommended that these nodules are considered for FNA at the 1 cm threshold [7].
Low Suspicion Nodules
Hyperechoic or isoechoic solid nodules or partially cystic nodules with an eccentric solid component have a lower suspicion for malignancy, 5–10 % [7]. Nodules in this category do not have any high risk features, including microcalcifications, extrathyroidal extension, or taller-than-wide shape. The threshold for FNA for these low risk nodules is 1.5 cm or larger [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree