MACIS score
20 year DSS (%)
<6
99
6–6.99
89
7–7.99
56
>8
24
Preoperative Assessment
The initial assessment of a patient with thyroid cancer begins with a focused history for intrinsic and acquired risk factors and a physical exam, including neck ultrasound, for local, regional, and metastatic disease. Review of pathology, indicated imaging, and the possibility of genetic analysis are also parts of the preoperative assessment.
Risk Factors
The main acquired risk factor for PTC is exposure to radiation, either external or internal. External radiation exposure is typically in the form of x-rays or radiation therapy for cancer or benign disease. Internal radiation exposure in the setting of radioactive particle ingestion is most commonly related to nuclear fallout (e.g., Chernobyl) or radioactive iodine treatment. The risk for developing thyroid cancer following radiation exposure is increased even with small doses, as low as 100 mGy. The relative risk of developing thyroid cancer is dose dependent initially (RR 6.8 with 2–4Gy), but then plateaus between exposures of 5–30Gy (RR14.8–15.2) and begins to decrease after 30Gy (RR 5.1–9.3) [15]. The risk is greatest in patients less than 15 years old and returns to almost baseline risk for exposures after age 20. Despite the increase in relative risk of developing thyroid cancer following radiation exposure, these cancers have similar recurrence rates following treatment (7–28 % in the short term) and similar long-term mortality (<1 % at 10 year) [16]. Therefore, a history of radiation exposure should warrant surveillance for thyroid cancer incidence but does not significantly impact prognosis or treatment [17].
The most common non-modifiable risk factors for PTC include family history, age, and sex. Familial inheritance of thyroid cancer is largely associated with medullary thyroid cancer ⎯ up to 25 % of medullary cancers are familial. However, there are some syndromes that have been linked to differentiated thyroid cancers. Familial adenomatous polyposis (FAP) is a syndrome primarily known for the development of innumerable colonic polyps that undergo malignant transformation over a short time period. Among the other extra-colonic malignancies associated with this syndrome are differentiated thyroid cancer, childhood hepatoblastoma, and medulloblastomas. These patients have a 2–12 % incidence of PTC (compared with 0.2 % in the general population), specifically a cribriform variant that is seen almost exclusively in FAP patients [18–20]. These cancers are often bilateral, occur in young female patients, and have similar prognosis to non-FAP-related PTC. Because of the increased incidence in this population and the low sensitivity of physical exam for detection, several studies suggest that screening thyroid ultrasound should be instituted in patients known to have FAP, although the age and interval to initiate this have not been determined [21, 22].
Other genetic syndromes, such as Cowden’s syndrome, Carney’s complex, and Werner’s syndrome, have an association with thyroid cancer, but they are largely linked to follicular thyroid cancer rather than papillary. Screening for thyroid cancer is recommended for these patients, although there is no documentation of inferior outcomes based on an underlying genetic mutation [23].
Familial non-medullary thyroid cancer (FNMTC), the majority of which are PTC, account for 3.2–6.2 % of thyroid cancers and follow an autosomal dominant pattern of inheritance. The diagnosis is established in patients with non-medullary thyroid cancer, in the absence of predisposing hereditary syndromes (e.g., FAP) or environmental risk factors (e.g., radiation exposure), and two or more first-degree relatives with thyroid cancer. If three or more family members are affected, it is 94 % likely that there is a familial predisposition [24]. Thyroid tumors of affected individuals are thought to have a more aggressive biology with increased incidence of multifocality and higher risk of recurrence [25, 26]. The data to support this is mixed with other studies suggesting no difference in pathophysiology compared to nonfamilial tumors [27]. A number of genes have been linked with FNMTC, most recently a germ line mutation in the HABP2 gene. This mutation was found in all members of a kindred with FNMTC and 4.7 % of a population of patients with sporadic thyroid cancer making it a strong candidate for a susceptibility gene [28]. Currently the recommendation is to perform a total thyroidectomy with strong consideration of CLND in patients with FNMTC. Screening is generally agreed upon for family members of affected patients beginning by age 20 or 5–10 years before the age of the youngest diagnosed member [29].
Thyroid cancer staging has always uniquely included age at diagnosis as a significant prognostic indicator of survival. The large majority of staging systems include age [1, 3–7, 30] as a risk factor with studies historically showing an improved survival associated with younger age at diagnosis. This finding has extended into the AJCC/UICC TNM staging system such that patients younger than 45 years old can only be maximally staged as stage II, regardless of the presence of distant metastatic disease (Table 11.2). The exact cutoff is somewhat arbitrary, typically around age 45 years, but most systems recognize age as a prognosticator of survival. The knowledge of thyroid cancer tumor cell biology does not provide a complete understanding of the contribution of age to survival, and it does not signify that young patients cannot have poor outcomes. In fact, young patients with metastatic disease have worse outcomes than older patients with localized disease. One study demonstrated a 50 % increase in mortality in patients from age 30 to age 40 years, and this increase continued to double for each 10-year increment thereafter. This finding translated into a rise in the overall risk of mortality for patients between stage I and stage II (HR 1.38, p = 0.2). When this is sub-stratified, the risk is ameliorated with older patients and exacerbated with younger patients (HR 11.48, p < 0.001) [31].
Table 11.2
AJCC/UICC TNM staging system for differentiated thyroid cancer
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Tumor ≤ 2 cm in greatest dimension, limited to the thyroid |
T1a | Tumor ≤ 1 cm, limited to the thyroid |
T1b | Tumor > 1 cm but ≤ 2 cm, limited to the thyroid |
T2 | Tumor > 2 cm but ≤ 4 cm, limited to the thyroid |
T3 | Tumor > 4 cm in greatest dimension Limited to the thyroid or with minimal extrathyroidal extension |
T4a | Moderately advanced disease Tumor of any size extending beyond thyroid capsule to invade subcutaneous soft tissue, larynx, trachea, esophagus, or RLN |
T4b | Very advanced disease Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
N1a | Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes) |
N1b | Metastasis to unilateral, bilateral, or contralateral cervical (levels I, II, III, IV, or V) or retropharyngeal or superior mediastinal lymph nodes (level VII) |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic group | |||
|---|---|---|---|
Under 45 years old | |||
Stage I | Any T | Any N | M0 |
Stage II | Any T | Any N | M1 |
45 years and older | |||
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 T1 T2 T3 | N0 N1a N1a N1a | M0 M0 M0 M0 |
Stage IVA | T4a T4a T1 T2 T3 T4a | N0 N1a N1b N1b N1b N1b | M0 M0 M0 M0 M0 M0 |
Stage IVB | T4b | Any N | M0 |
Stage IVC | Any T | Any N | M1 |
Additional studies have investigated the specific age at which patients are separated into low and high risk. A recent study suggested that an age of 55 years rather than 45 years may be a more accurate predictor of disease-specific survival (DSS). For localized and metastatic disease, age was the most significant predictor of outcome, with age cutoffs of 56 and 54 years, respectively [32]. Similarly, another study investigating the effect of age and gender on DSS showed that men and women older than 55 years had similar outcomes, while women diagnosed at an age less that 55 years had improved outcomes over men [33]. Therefore, while one can be confident that age does affect survival, the relationship is complex and likely involves additional factors that have not been fully identified or quantified at this time.
Male gender has long been considered a risk factor for developing more aggressive thyroid cancer. The incidence of PTC in women is three times that of men, and men present with more advanced tumors leading to inferior outcomes [34]. However, when further evaluated, women tend to present at a younger age and with earlier-stage tumors, so when matched by stage, men have similar outcomes to women. Additionally, when survival curves for men and women are compared to the general population, the female advantage seen in thyroid cancer outcomes mimics the survival advantage seen in the general population. When a cohort of 3572 patients was separated into those younger and older than 55 years, females younger than 55 had better DSS for PTC compared to men; however, the outcomes were similar between genders when evaluating patients older than 55 years [33]. One explanation for this is the theory of estrogen modulation of tumor biology, in which more frequent but less aggressive tumors develop during the period of estrogen exposure. Conversely, more aggressive tumors may develop in postmenopausal women [35]. There are obviously multiple factors that contribute to tumor biology and prognosis in different populations, and the excellent overall long-term survival of thyroid cancer patients makes it difficult to study treatment effects. It is currently accepted that risk factors are taken into consideration in patient management, but there are no specific guidelines for changes in treatment or surveillance.
In addition to screening for risk factors for development of PTC, patients should be asked about physical findings that would increase their risk of malignancy ⎯ rapid growth of a solitary nodule or compressive symptoms (dysphagia, dysphonia, cough/dyspnea, hoarseness). These symptoms are not diagnostic of malignancy, but their presence would increase clinical suspicion and warrant timely investigation.
Physical Examination
The physical examination for suspicious or confirmed thyroid cancer historically has relied upon manual palpation of the thyroid gland and regional lymph nodes. However, examination has expanded over time to include ultrasound of the neck (performed in clinic) and laryngoscopy. Direct examination of the thyroid should focus on the consistency of the gland, fixation to adjacent structures, and size of the nodule ⎯ nodules measuring greater than 4 cm have a 26 % chance of harboring a clinically significant thyroid cancer [36]. Fine-needle aspiration (FNA) is the recommended method of further evaluating thyroid nodules. It can be performed by palpation or under ultrasound guidance. The Bethesda system provides a framework with which to interpret FNA results, and if cytology is suspicious for or diagnostic of PTC, the patient should go on to have surgical management [37].
A thorough evaluation for enlarged cervical lymph nodes should cover levels II through VI [4]. Levels II–IV are found adjacent to and around the carotid sheath, often posterior to the sternocleidomastoid muscle, with II between the level of the base of the skull and hyoid, III being between the level of the hyoid and cricoid cartilage, and IV being between the cricoid cartilage and the clavicle. Level V consists of the transverse cervical chain and posterior triangle lymph nodes, and level VI comprises the central compartment between the carotid sheaths transversely and the hyoid and sternal notch longitudinally (Fig. 11.1). Lymphadenopathy is common in PTC ⎯ it is grossly evident in 20–50 % of cases and microscopically presents in up to 90 % [37]. Despite the necessity of the manual exam, there is a high degree of variability in exam findings even between specialists, which is improved significantly with the use of ultrasound [38].
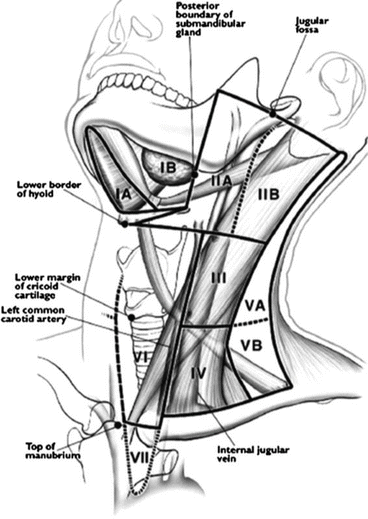

Fig. 11.1
Neck lymph node levels and compartments (With permission from Elsevier [89])
Preoperative Imaging
The use of ultrasonography significantly improved the ability of clinicians to assess for lateral and central compartment lymphadenopathy in several studies ⎯ detecting 14–20 % additional lymph nodes not detected on physical exam for patients undergoing primary surgery and 28–64 % in patients having re-operative surgery [39, 40]. The finding of non-palpable cervical chain lymph nodes on ultrasound leads to a change in the operative plan in 40 % of patients [39]. Although ultrasound cannot distinguish definitively between benign and malignant characteristics of nodules or lymph nodes, there are several findings that increase the suspicion of malignancy. Hypoechogenicity, microcalcifications, irregular margins, increased blood flow, and the absence of a halo have median sensitivities and specificities ranging from 52 to 81 % and 53 to 83 %, respectively, for malignant thyroid nodules. Sonographically, metastatic lymph nodes characteristically have a larger ratio of short to long axis (making the nodes rounder), a hyperechoic cortex, intranodal calcifications and cystic necrosis, loss of echogenic hilum, and a peripheral or diffuse vascular pattern [41]. Suspicious lymph nodes that are identified clinically or on imaging should be biopsied with ultrasound-guided FNA and sent for cytology or thyroglobulin levels. Preoperative lymph node mapping is considered the standard of care for patients having surgery for thyroid malignancy [37].
In the past, preoperative laryngoscopy was reserved for patients presenting with complaints of voice abnormalities or clinical evidence of tumor invasion; however, several studies have shown poor correlation between patient reporting and confirmed vocal cord dysfunction [42, 43]. And while surgeon assessed voice abnormalities and a patient history of neck surgery are reasonable starting points for establishing who should have laryngoscopy, it is advocated by many that it should performed in all thyroid cancer patients preoperatively [43, 44]. Ultrasound assessment of vocal cord function may be another screening tool easily performed in the office to help select those patients for whom laryngoscopic visualization of the vocal cords is necessary [45–47].
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended as routine modalities for preoperative imaging of the thyroid. Ultrasound is less expensive and accurate and avoids radiation exposure and is therefore generally sufficient in preoperative staging. However, there are instances when cross-sectional imaging with CT or MRI may contribute to clinical decision making, such as when there is physical evidence of invasion, incomplete evaluation of a large tumor or bulky nodes seen on ultrasound (e.g., tumor/node extension into the mediastinum), or any question about anatomic extent of disease [48]. The utilization of enhanced CT scanning preoperatively may delay the use of RAI treatment for several weeks postoperatively due to the iodine load of intravenous contrast. However, contrasted CT scans are necessary to avoid an incomplete resection; therefore, the benefit of a good preoperative imaging and planning may outweigh the mild potential delay in postoperative radioactive iodine treatment.
Positron emission tomography (PET) or PET-CT is typically reserved for evaluating patients for persistent or recurrent disease and non-RAI avid disease; its role is not established as a primary preoperative imaging modality [48]. It is most useful to assess for recurrent disease or as part of a surveillance program, and its findings have been shown to alter management in up to 30 % of recurrent cases [49].
Histopathology
Preoperatively, FNA is performed to obtain a cytological diagnosis of thyroid cancer. Papillary thyroid cancer has several typical findings that are not pathognomonic but are frequently present. These include the presence of (1) papillae formed by layers of tumor cells; (2) large, overlapping, oval-shaped nuclei containing hypodense chromatin and cytoplasmic inclusion bodies (Orphan Annie eye nuclei); (3) nuclear grooves; and (4) psammoma bodies. The impact of the pathological diagnosis becomes more important when variants of PTC are identified; however, these are usually only recognized on postoperative histology. Variants associated with a poorer prognosis include tall cell, diffuse sclerosing, and insular variants. Others with a more favorable prognosis include follicular, solid, trabecular, oncocytic, microfollicular, pseudo-Warthin, and clear cell variants [50]. The incidence of the tall cell variant ranges from 1 to 19 % of PTC, and histologically this subset lives up to its name in that 30 % of cells are twice as long as they are wide. Diffuse sclerosing variant has an incidence of 2–6 %, and in addition to classic PTC features, it exhibits stromal fibrosis, lymphocytic infiltration, and squamous metaplasia. These two variants were described in a paper by Kazaure et al., which reviewed outcomes of over 43,000 cases of PTC from the SEER database. They showed increased incidence of extrathyroidal extension, multifocality, nodal and distant metastases in tall cell, and diffuse sclerosing variants when compared to classic PTC (Table 11.3). Additionally, there was a significant decrease in 5-year disease-specific survival between tall cell and classic PTC (87.5 vs. 97.4, p < 0.001) [51]. Insular variants also have an inferior prognosis compared with classic PTC. These variants are described as existing in “nests” or insulae of tumor cells. They typically form large tumors and are prone to extrathyroidal extension (47 %), lymph node metastases (62 %), and distant metastatic disease (30 %). These patients have a decreased 5-year DSS compared with classic PTC (73 vs. 97 %, p < 0.001) [52].
Table 11.3
Pathologic characteristics of 43,738 patients with aggressive variants and classic PTC, SEER (1988–2008)
Characteristic | DSV (n = 261) | TCV (n = 573) | Papillary (classic) (n = 42,904) | P |
|---|---|---|---|---|
Tumor size (cm), mean (SEM)a | 1.7 (1.0) | 2.7 (0.8) | 1.8 (0.1) | <0.001 |
≤1.0 | 46.3 | 16.5 | 36.2 | |
1.1–2.0 | 27.5 | 28.8 | 31.4 | |
2.1–4.0 | 15.8 | 35.9 | 25.2 | |
>4.0 | 10.4 | 18.8 | 7.2 | |
Focality: multifocal | 29.5 | 29.3 | 26.7 | <0.001 |
Extrathyroidal extension | 31.0 | 54.7 | 20.1 | <0.001 |
≥1 positive lymph nodeb | 72.2 | 66.8 | 56.3 | <0.001 |
Median (IQR) | 4 (1–11) | 3 (1–7) | 3 (1–7) | |
SEER stagec | <0.001 | |||
Localized | 48.1 | 28.3 | 58.2 | |
Regional | 44.6 | 60.6 | 37.5 | |
Distant | 7.3 | 11.1 | 4.3 | |
5-year overall survival | 87.5 | 80.6 | 93.5 | <0.001 |
5-year disease-specific survival | 96.1 | 87.5 | 97.4 | <0.001 |
Molecular Profiling
The preoperative assessment for papillary thyroid cancer has recently expanded from a detailed history, physical exam, and imaging to include molecular profiling. In the last two decades, many advances have been made that identify genetic mutations present in cancer with several specific mutations that are found frequently in thyroid cancer. The most frequently discussed mutation is BRAF, an isoform of the RAF (rapidly accelerated fibrosarcoma) gene. It is mutated in papillary thyroid cancer in 30–80 % of cases [53, 54]. The BRAF protein acts in the RAF/MEK/MAPK pathway and, when mutated, forms the BRAFV600E protein, which is constitutively activated. The BRAF mutation has been described in melanoma and colon cancer, but is most frequently associated with PTC. Over the last 10 years, there have been a number of studies associating BRAFV600E with invasive growth patterns, increased risk of recurrence, and higher mortality [55, 56]. However, there have also been several studies that have failed to replicate these findings and support a less aggressive tumor biology associated with mutation [57, 58]. There is also some evidence that unless there is a clinically relevant change in management based on BRAF status, the financial implications of genetic analysis might outweigh any benefit [59].
Other genes implicated in PTC that also play a role in the MAPK activation pathway are RET and RAS. The RET gene undergoes rearrangements leading to chimeric genes that are constitutively active; these are referred to as RET/PTC. Germ line mutations in RET/PTC are the cause of medullary thyroid cancer in MEN2 syndromes; however, this gene product is not expressed in normal thyroid cells, which explains why PTC is not seen in MEN2 patients. Somatic rearrangements associated with PTC are present in approximately 40 % of sporadic cases [60, 61]. RAS mutations are most commonly found in follicular adenomas, suggesting that these mutations may lead to a more benign phenotypic outcome; however, they have been described in follicular variants of papillary thyroid cancer [62].
Recently, Yip et al. reported on the correlation of clinical outcomes with specific genetic mutations in a study of 1510 patients with thyroid cancer (97 % PTC). They showed that patients with a RAS mutation had a low incidence of extrathyroidal extension (4.6 %) and lymph node metastases (LNM) (5.6 %) compared to patients with a BRAFV600E mutation that had significantly higher incidences (extrathyroidal extension 51 %, LNM 46 %). Additionally when grouped into histologically similar tumors, BRAFV600E and RET/PTC mutations were associated more often with stage III/IV disease (40 vs. 15 %, p < 0.001) and recurrence (10 vs. 0.7 %, p < 0.001) compared with tumors containing RAS or PAX8/PPARG mutations [63]. Some of the newer gene sequencing assays have been reported to have a high sensitivity (90.9 %) and specificity (92.1 %) for detecting gene mutations and classifying thyroid cancers [64]. The potential to characterize the molecular identity of tumors preoperatively may help to guide management of individuals based on tumor biology.
Intraoperative Assessment
Thyroid Gland Assessment
The main objectives intraoperatively are to perform an appropriate oncologic resection and to minimize the risk of postoperative complications. The thyroid gland and cervical lymph nodes are assessed preoperatively, but the accuracy of determining specific characteristics such as extrathyroidal extension of the primary tumor or metastatic lymph node deposits is limited (sensitivity and specificity for each was 60 and 80 % for extrathyroidal extension and 83 and 87 % for lymph node metastasis [40, 65]). Gross invasion of tumor into the strap muscles necessitate an en bloc resection of the involved muscles. Suspicion of tracheal invasion not predicted preoperatively can be difficult to confirm and may require frozen sections to establish whether resection is warranted. The reported positive and negative predictive values of frozen section evaluation of invasion were 98 % and 87 %, respectively. This works well when extrathyroidal extension is detected but runs the risk of performing an inferior resection for 13 % of patients when it is not detected [66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree