Study
Year
Total subjects (n)
rhTSH (n)
THW (n)
Definition of successful ablation
Length of follow-up
Successful ablation from rhTSH (n)
Successful ablation from THW (n)
p
Lee et al. [73]
2010
291
69
222
Negative WBS, negative neck US and Tg < 1.0 ng/ml
12 months
63 (91.3 %)
203 (91.4 %)
0.2061
Mallick et al. [36]
2012
421
210
211
Negative WBS and TG <2.0 ng/ml
6–9 months
183 (87.1 %)
183 (86.7 %)
0.26
Molinaro et al. [37]
2013
120
70
50
Negative neck US, stimulated Tg < 1.0 ng/mL, and negative Tg-Ab
10 years
47 (67.1 %)
37 (74 %)
0.53
2005
63
32
28
Negative WBS (no uptake)
8 months
24 (75 %)
24 (85.7 %)
0.300
Schlumberger et al.
[35]
2012
684
348
336
Negative neck US and Tg < 1.0 ng/mL
6–10 months
319 (91.7 %)
312 (92.9 %)
Not stated
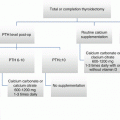
The benefits of using rhTSH include improved quality of life due to less frequent symptoms of hypothyroidism, including fatigue, lacrimation, constipation, weight gain, and cold intolerance. However, within 3 months after ablation, patients treated with either THW or rhTSH have a similar quality of life [38]. Other studies have shown that patients may be able to receive less overall radiation with rhTSH use, as the renal clearance of 131−I is faster in patients treated with rhTSH use compared with THW [29, 39]. Additionally, some suggest that using the THW method exposes the patient to prolonged duration of TSH elevation, which may promote tumor growth [31, 40]. Furthermore, even after weeks of withdrawal from LT4, adequate elevation of TSH may not be achieved due to endogenous production of thyroxine from the thyroid remnant or residual disease [41].
The limitations of rhTSH include its cost and availability, as many centers may not have access to it. In the past, some insurance companies also would not reimburse for rhTSH, but it is now easier to have rhTSH pre-authorized for patients. Side effects of rhTSH include mild headaches and nausea [31]. However, as with the withdrawal method, rhTSH can stimulate tumor growth, which may result in compression of adjacent structures, respiratory compromise, or neurologic dysfunction [41]. Furthermore, in patients with high-risk DTC or with extensive lymph node involvement, rhTSH is currently not routinely recommended for most patients due to insufficient studies measuring the risks of morbidity and mortality associated with rhTSH in this cohort of patients [19].
Pre-RAI Treatment Nuclear Imaging
Historically, a whole body 131I or 123I scan prior to radioiodine ablation has been used to calculate the amount of residual thyroid tissue present following thyroidectomy, detect functional metastatic disease, evaluate if treatment with 131I is warranted, or assess if a two-step ablation is required [42, 43]. The ATA also recommends performing pretreatment 131I scans in those patients for whom the extent of thyroid remnant cannot be ascertained from the surgical operative report or neck ultrasound, or in whom the scan would potentially change management [18]. However, some practitioners have shifted away from the use of pretreatment scans because it is thought that there may be potential “stunning” of thyroid tissue with pretreatment iodine [43]. Studies suggest that stunning occurs due to downregulating transcription of NIS on thyroid cells [44]. Additionally, some studies suggest that although stunning may not be apparent in the posttreatment scan, patients who have had a pretreatment scan have decreased successful ablation rates [45]. Others have discontinued their use of pretreatment scans using the rationale that the posttreatment scan is more sensitive to detect distant metastatic disease.
However, some retrospective studies suggest that the utility of pre-ablation scans is still clinically relevant, as it may alter management. For example, if a pretreatment scan shows lymph node involvement with focal uptake, a higher RAI dose may be considered. One retrospective trial reported that the management in more than 50 % of patients (total n = 355) was altered based on pretreatment 131I scanning results, including 18 % with either local or distant metastatic disease, 6 % with no focal uptake, and 14 % with lymph node metastases [42]. Another study retrospectively examined patients who received pretreatment 123I scans. Of 122 patients, the pretreatment scan altered management in 25 %. The pretreatment scan was able to determine if a large remnant remained and was better able to differentiate between remnant tissue and lymph node disease as well as identify iodine-avid foci away from the midline (i.e., metastatic disease) [43].
Radioactive Iodine Dosing
As with other cancers, it is important to use the lowest effective dose of radiation to ablate the thyroid remnant in order to reduce the risk of secondary cancers. In patients with high-risk features, such as vascular invasion or known metastatic disease, it is generally accepted to use a higher dose of 131I as a form of adjuvant therapy. In intermediate-risk cancers, there has been shown to be up to a 29 % reduction of recurrence with use of RAI [27]. The ATA currently recommends using a lower dose of 30 mCi for low-risk or intermediate-risk disease with low-risk features [19]. However, the dose of 131I used to treat intermediate papillary thyroid cancer is controversial. A retrospective study in Korea treating intermediate-risk patients with tumors <2 cm with microscopic extrathyroidal extension with either low- or high-dose RAI ablation found similar rates of successful ablation; there was no difference in recurrence rates between the two groups at follow-up intervals of up to 5 years [46]. However, another large retrospective trial of 1298 WDTC patients showed that patients older than 45 years of age had increased mortality with lower doses than with higher doses of RAI [4, 47].
Additional trials similarly show mixed results regarding whether there is a benefit to higher doses of RAI (Table 20.2). Fallahi and colleagues reported better ablation success rates with higher doses of RAI [48]. This study also noted that patients who received a low dose of RAI often required a second ablation dose, which ultimately resulted in higher cumulative dose of RAI. However, Maenpaa et al. reported similar ablation success rates in patients treated with 30 mCi or 100 mCi and no difference in recurrence rates when these patients were followed for a median of 51 months [49]. As mentioned previously, recent large studies in the United Kingdom and France showed that ablation rates were similar with 30 mCi or 100 mCi of 131I [35, 36]. Similarly, there was no difference shown between low- or high-dose regimens of RAI in several other studies [46, 50, 51].
Table 20.2
Comparison of ablation rates from high-dose RAI (≥50 mci) vs. low-dose RAI (<50 mci) for treatment of WDTC
Study | Year | Total subjects (n) | Number receiving high-dose RAI ≥ 50 mCi (n) | Number receiving low-dose RAI < 50 mCi (n) | Definition of successful ablation | Length of follow-up | Successful ablation from high-dose RAI (n) | Successful ablation from low-dose RAI (n) | p |
|---|---|---|---|---|---|---|---|---|---|
Caglar et al. [50] | 2012 | 108 | 55 | 53 | Negative neck ultrasound, negative WBS, serum Tg < 0.2 ng/ml | 6–12 months | 35 (64 %) | 32 (60 %) | Not significant |
Fallahi et al. [48] | 2012 | 341 | 170 | 171 | Negative WBS and serum Tg < 2.0 ng/ml and anti-Tg < 100 IU/ml | 12 months | 117 (68.8 %) | 71 (41.5 %) | Not stated |
Han et al. [46] | 2014 | 176 | 80 | 96 | Negative stimulated Tg, neg Tg Ab, and absent remnant thyroid on US | 12 months to 7 years (retrospectively) | 72 (90 %) | 93 (97 %) | 0.75 |
Maenpaa et al. [49] | 2008 | 151 | 77 | 81 | Negative WBS and Serum Tg < 1 ng/mL | 4–8 months | 43 (56 %) | 42 (52 %) | 0.61 |
Mallick et al. [36] | 2012 | 421 | 207 | 214 | Negative WBS and Tg < 2.0 ng/ml | 6–9 months | 184 (88.9 %) | 182 (85 %) | 0.24 |
Pilli et al. [51] | 2007 | 72 | 36 | 36 | Negative WBS and serum Tg < 1 ng/ml | 6–8 months | 29 (80.6 %) | 31 (86.1 %) | Not stated |
Schlumberger et al. [35] | 2012 | 684 | 337 | 347 | Negative neck US and Tf < 1.0 ng/ml | 6–10 months | 307 (91.1 %) | 331 (93.5 %) | Not stated |
In patients with high-risk papillary thyroid cancer, such as those with distant metastatic disease or macrovascular invasion, higher doses of RAI are generally used [52, 53]. However, the optimal dose for these patients is still unknown. Some studies have showed no significant benefit of a higher dose, as well as increased morbidity related to RAI use [54]. There are retrospective studies that do suggest that even in patients with high-risk disease, such as a tumor >4 cm in size or lymph node metastases, may do just as well with a low dose (e.g., 30 mCi) of RAI [55].
Posttreatment Follow-Up
About 1 week after radioactive iodine is given, the patient should undergo a posttreatment uptake whole body scan (WBS). This scan informs the practitioner of focal iodine uptake by the thyroid remnant and any residual tumor, as well as discloses the location of iodine-avid metastatic disease [17, 56]. If there is no uptake in the thyroid bed, this suggests that the tumor is not iodine avid and other treatments should be pursued.
Subsequent to the initial ablation, patients should be followed for the measurement of serial serum Tg concentrations and Tg antibodies titers and neck ultrasonography. Neck ultrasonography with a concurrent stimulated Tg and Tg antibody is recommended 6–12 months after ablation to determine ablation success [19]. If ablation is successful, the stimulated thyroglobulin level should be less than 1 ng/mL, and the serum thyroglobulin antibody should be negative [19, 35, 37]. Some practitioners will also obtain a WBS 6–12 months after ablation to determine if ablation is successful. In this case, ablation is deemed successful if there is less than 0.1 % uptake on the posttreatment WBS with a concurrent stimulated (either with THW or rhTSH) serum Tg level less than 1–2 ng/mL [35, 36, 50].
What Patients Should Expect After Receiving RAI
Although therapy with RAI is well tolerated by most patients, there are certain aspects of therapy that should be discussed with patients prior to treatment. Patients should be informed that the radioactivity is excreted in their bodily fluids and should therefore drink sufficient fluid after receiving a dose of radioactive iodine. Additionally, patients must also be counseled regarding the recommended physical proximity to loved ones in the immediate posttreatment phase. Patients with young children or close relationships with pregnant women should be advised to refrain from interacting with their loved ones for the immediate few days and even weeks [57]. Because this may not be feasible for patients in certain instances, many centers hospitalize patients for radioactive iodine therapy. These patients are monitored in the hospital; once radioactivity has decreased to a level that is deemed safe, patients are discharged [57, 58].
Complications of Treatment with RAI
Treatment with 131I is generally well tolerated by most patients. However, as with all medical therapies, there are side effects and complications with 131I, including salivary gland dysfunction, infertility, and possibly secondary malignancy (Table 20.3) [7, 59, 60]. Immediately following 131I administration, patients may experience salivary gland dysfunction, which may occur in up to 30 % of patients [61]. Iodine is concentrated in salivary glands at 7–700 times the concentration in serum, thus increasing the risks of salivary gland dysfunction following RAI [61, 62]. Salivary gland dysfunction can be temporized with the use of sour candies to stimulate the salivary glands. Patients can also report dysgeusia, nausea and vomiting, nasal irritation, or stomatitis. Cytopenias have also been reported and usually present as a subclinical lowering of leukocytes and/or platelets [25, 61]. These effects typically wear off as the kidneys excrete 131I and are self-limited.
Table 20.3
Potential risks of RAI
Short-term risks | Long-term risks |
|---|---|
Nausea | Chronic sialadenitis |
Vomiting | Xerostomia |
Transient sialadenitis | Secondary malignancies: Leukemia Breast cancer Testicular cancer Salivary duct cancer |
Change in taste/smell | Infertility |
Headache | Delayed childbearing |
Bone marrow suppression | |
Transient ovarian failure | |
Transient testicular failure | |
Epistaxis |
However, there are potential chronic side effects, including chronic sialadenitis, xerostomia, nasolacrimal duct dysfunction, prolonged bone marrow suppression, and earlier menopause [61, 63–65]. There have also been reports of delayed childbearing and infertility [60, 64, 66, 67]. RAI treatment for thyroid cancer has been associated with increased risk for other primary malignancies, including leukemias, renal cell carcinoma, salivary cancers, and breast cancer [7, 64, 68, 69]. In younger patients (those aged less than 25 years old at time of treatment), RAI has been associated with a 1.42 relative risk of malignancy, with the most commonly reported malignancies being renal cell carcinoma, salivary cancer, and leukemia [7]. In a recent meta-analysis of US and European studies, Sawka et al. reported a 1.19 relative risk of developing a second primary malignancy after RAI among adults using a minimum latency of 2–3 years after the thyroid cancer diagnosis [69].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree