First author and publication date
Recurrence-free rate after surgery ± RAI and EBRT (%)
Recurrence-free rate after surgery ± RAI(%)
Tubiana (1985) [12]
86
79
Farahati (1996) [13]
90
50
Tsang (1998) [14]
93
78
Chow (2002) [15]
88
84
Kim (2003) [16]a
95
63
Phlips (2004) [17]
97
79
Brierley (2005) [18]b
86
66
Keum (2006) [19]c
89
38
Azrif (2008) [6]a
81
Not reported
Schwartz (2009) [7]d
79
Not reported
Although lacking proof from randomized controlled studies, EBRT appears to benefit patients at high risk of local recurrence. Risk is usually defined by older age, extrathyroidal extension, and microscopic residual disease. It is our current institutional policy to recommend EBRT in older patients (usually over 50 years of age) with gross extrathyroidal extension noted at the time of surgery that invades posteriorly into the trachea-esophageal groove. Invariably, there is a risk of microscopic disease after surgery in this region, unless an extirpative operation such as a laryngectomy was performed. We believe such disease is unlikely to be controlled by radioactive iodine alone. It is important to involve the surgeon in the discussion to ensure that the extrathyroidal extension was significant and that salvage surgery would be difficult if not impossible without extensive radical surgery, and that recurrence would have a significant impact on speech, swallowing, and quality of life. Patients with gross extrathyroidal extension that invades anteriorly into the strap muscles can usually be resected with clear margins and do not require EBRT. There may also be a role for EBRT in patients who have repeated surgery for recurrent cervical nodal involvement and in whom further surgery may result in unacceptable risks or complications.
Radiation Therapy
If patients are to have RAI and adjuvant EBRT, our preference is usually to give 131I and then perform postoperative CT scans after the post-RAI therapy scan and reassess the extent of disease after surgery, as seen on post-RAI scan and CT scan. A PET scan if available may provide additional information. Although in theory EBRT could reduce the effectiveness of RAI, there is no good evidence to support this, and therefore if there is concern about the extent of local disease that may cause an oncological emergency without control of that disease, such as gross residual disease after spinal cord decompression, then we will give EBRT before RAI.
A preoperative CT scan with intravenous contrast is a great aid in planning any postoperative radiation therapy. In our institution, surgeons routinely perform CT scans with intravenous contrast in any patients with a large mass, fixation, pain, or hoarseness that suggest a possible T4 tumor. Past concerns that iodinated contrast may interfere with the effectiveness of RAI have been allayed with modern water-soluble contrast media (see Chap. 6). A 1 or 2 months delay only is now required for the urinary iodine levels to fall after a contrast enhanced CT [21]. An alternative to CT with contrast is a cervical MRI; however, the ability to identify laryngeal cartilage or lymph node involvement may be inferior.
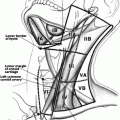
It is our practice to include in the volume to be irradiated the surgical thyroidectomy bed and levels III, IV, and VI and part of level V. The volumes extend from the hyoid bone superiorly to the aortic arch inferiorly. Sixty Gray in 30 fractions is usually prescribed to the thyroid bed and areas of surgical dissection if there is concern for microscopic residual disease, and a lower dose of 54 Gy in 30 fractions to undissected areas at risk of microscopic disease. In patients with gross residual disease, unresectable or unresected disease, a higher dose of 66 Gy in 33 fractions to 70 Gy in 35 fractions is given to the gross disease, with 56 Gy in 33 or 35 fractions to the areas at risk of microscopic disease (Fig. 26.1). For patients with unresected gross disease and poor performance status unable to tolerate 66 Gy, occasionally we will give 50 Gy in 20 fractions over 4 weeks. For palliation in patients with local symptoms and disseminated disease and limited performance status radiation such as 20 Gy in 5 fractions or 30 Gy in 10 fractions may be prescribed.
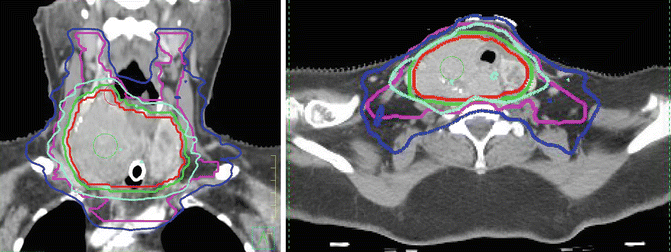

Fig. 26.1
A 76-year-old woman presents with a short history of shortness of breath but a long-standing history of a thyroid mass. Biopsy shows differentiated thyroid cancer. CT scan is reported as extensive thyroid mass with tracheal involvement and involvement of the carotid. The tumor is considered unresectable. She is referred for EBRT. A tracheostomy is performed and she is prescribed 70 Gy in 35 fractions over 7 weeks. Sagittal and axial view of radiation treatment plan. The red line is the gross target volume (GTV). The lime green line is the 70 Gy isodose line (all the tissue with that volume described by the 70 Gy isodose line received a minimum of 70 Gy). The dark blue line is the 56 Gy isodose line
Volume and Toxicity
There is controversy on the appropriate volume to treat with EBRT. Our philosophy has been to give EBRT to control disease in the thyroid bed and tracheoesophageal groove. Consequently our volumes are smaller than in other institutions that treat the entire cervical nodal basin (levels II to V and some include the retropharyngeal lymph nodes). Although this volume may result in fewer nodal recurrences (which hopefully can be salvaged with further surgery), the radiation-induced acute and late toxicity will be greater. Minimizing toxicity from EBRT is critically important, especially in patients who are already at risk from xerostomia because of RAI. Both acute and late effects following EBRT are dependent on the volumes radiated as well as the dose to normal structures. Structures of particular concern include the parotid glands, radiation to which potentiates the risk of xerostomia. Other volumes of concern include the pharyngeal constrictors which may result in dysphagia and, in rare circumstance, feeding tube dependence, and the mandible which is associated with a risk of osteoradionecrosis, although this is rare. Second malignancy can occur after any radiation treatment but is exceedingly rare and has not to our knowledge been reported following EBRT for thyroid cancer but should always be a consideration especially in treating young patients. Not surprisingly in a study on quality of life in patients treated for thyroid cancer, those who had EBRT and RAI fared less well than those who had RAI only after surgery [22]. Intensity-modulated radiation therapy (IMRT, Table 26.2) which is now the standard for treating head and neck cancers in many centers, has been reported to be associated with less late morbidity than older techniques [7].
Table 26.2
Glossary of terms used in radiation therapy
Intensity-modulated radiotherapy (IMRT). The intensity of the radiation and the shape of the radiation fields are varied (modulated) so that the radiation is conformed (or tailored) to the target to be treated. The toxicity to normal structures is reduced and therefore a higher radiation dose can potentially to be given to the cancer |
Radiosurgery and stereotactic body radiotherapy. This is highly conformed precision radiation. A single large fraction of radiation is given |
Stereotactic radiosurgery (SRS). SRS usually refers to radiosurgery (a single large fraction of radiation) to the brain (Gamma Knife © is an example of SRS) |
Stereotactic body radiosurgery (SBRS). SBRS usually refers to radiosurgery that is administered to parts of the body other than the brain (CyberKnife © and X-Knife © are examples) |
Stereotactic body radiotherapy (SBRT). SBRT usually refers to highly conformal radiotherapy given in 3–6 fractions rather than a single fraction |
In general however well-planned EBRT results in acceptable levels of acute toxicity. Serious complications are rare and EBRT does not preclude future surgical intervention by surgeons with experience in operating in previously irradiated tissues. Acute toxicity that occurs toward the end of a course of radiation therapy includes moderate skin erythema, and rarely moist desquamation and mucositis of the esophagus, trachea, and larynx. Larger radiation volumes to treat nodal areas extending to the salivary glands result in changes in taste, sensation, and xerostomia. Late toxicity is uncommon, and may comprise skin telangiectasias, skin pigmentation, soft tissue fibrosis, and mild lymphedema, usually appearing just below the chin. Esophageal stenosis in our experience can usually be treated by dilatation. We have not seen G-tube dependency or tracheal stenosis in our patients, although it has been reported by others [7]. Two large series on the use of EBRT reported no radiation therapy oncology group grade IV toxic effects [13, 14).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree