Fig. 2.1
Association between type 2 diabetes mellitus and risk of hip fracture in case–control and cohort studies. Each square shows the study-specific relative risk (RR) estimate (the size of the square reflects the study-specific statistical weight, that is, the inverse of the variance), and the horizontal line shows the related 95 confidence interval (CI). The diamond shows the summary RR estimate, and its width represents the corresponding 95 % CI. All statistical tests were two-sided. Statistical heterogeneity between studies was assessed with Cochran’s Q test. Reprinted with permission from Janghorbani et al. [26]
Interaction with Age But Not Gender
There is limited evidence that the relationship between diabetes and hip fracture may be stronger at younger ages. A study in Manitoba, Canada, reported an increased rate of hip fracture with diabetes in those <65 years old (6.27; 95 % CI 3.62–10.87) that was greater (p for interaction = 0.002) than the relative rate in those ≥65 years old (2.22; 95 % CI 1.71–2.90) [27]. A study of diabetes and hip fracture in Taiwan reported a similar interaction between diabetes and age [20]. For gender, the association between diabetes and hip fracture appears to be similar for women and men. In the meta-analysis by Janghorbani et al., the association between T2D and hip fracture did not differ by gender (p for interaction = 0.51). The summary relative risk was 2.1 (1.6–2.7) among women (eight studies), and 2.8 (1.2–6.6) among men (five studies) [26]. Similarly, in the Canadian cohort, there was no evidence of interaction by gender for diabetes and hip fracture [27].
Incidence of Any Fracture and Type 2 Diabetes
The two meta-analyses discussed earlier provided evidence of a modest increase in the risk of any fracture with T2D. As with hip fracture, studies of all fractures that have adjusted for the higher BMD associated with type 2 diabetes have generally found that those with T2D have an elevated risk for a given BMD. Vestergaard reported an age-adjusted relative risk of 0.96 (0.57–1.61), combining five studies, with strong evidence of heterogeneity (p < 0.01) [14]. When two studies reporting reduced fracture risk with T2D were excluded, heterogeneity was reduced (p = 0.88), and the estimated relative risk indicated a modest increase in the risk of any fracture with T2D (1.19; 95 % CI 1.11–1.27). Janghorbani et al. reported an increase in non-vertebral fractures with T2D in results from eight studies, adjusted for BMI and/or BMD (adjusted RR 1.2; 95 % CI 1.01–1.5) [26]. Since these meta-analyses, the WHI study reported an increased risk of any fracture in women (age-adjusted RR 1.29; 1.20–1.38) [17], and a similar increased risk was reported for Rochester MN residents (Standardized incidence ratio = 1.3; 1.2–1.4) [16]. In a cohort of older US men, risk of non-vertebral fracture was not increased in age-adjusted models (HR = 1.12; 0.94–1.34) but was modestly elevated after adjustment for BMD (HR = 1.30; 1.09–1.54) [28].
A few studies have considered whether diabetes interacts with age, gender, or race for the outcome of any fracture. A recent study in the United States using NHANES data reported an interaction (p < 0.05) between diabetes and race for the outcome of non-skull fracture, comparing age- and sex-adjusted results for the relative rate of fracture associated with T2D in Mexican-American (HR = 2.29; 1.41–3.73), non-Hispanic black (1.86; 1.05–3.30), and non-Hispanic white (HR = 1.17; 0.89–1.52) participants [29]. The study found no evidence of interaction between diabetes and age or gender for the outcome of non-skull fracture. In WHI there was a suggestion of an increased relative rate of any fracture in black women (HR = 1.33; 1.00–1.75) compared with non-Hispanic white women (HR 1.18; 1.08–1.29) [17]. In a smaller cohort of older black and white adults, there was no evidence of interaction between diabetes and gender or race for the outcome of any fracture [30].
The data available for specific fracture sites other than hip are more limited. Janghorbani et al. summarized results for studies published before 2006. With the exception of distal forearm fracture (summary RR = 0.98; 95 % CI 0.8–1.2), the point estimates for the summary relative risks were modestly elevated (ankle 1.3, proximal humerus 1.3, vertebra 1.2, foot 1.3), but only the RR for foot fracture was statistically significant. Studies published since this meta-analysis with results for specific non-hip sites include the WHI cohort [17] and Rochester, Minnesota population [16]. Results from WHI were quite similar to the Janghorbani et al. meta-analysis with increased rates for all fracture sites considered with the exception of the lower arm/wrist [17]. Many vertebral fractures do not come to clinical attention, but can be identified on spine X-rays as morphometric vertebral fractures. Some studies of T2D and morphometric vertebral fractures have reported increased prevalence of vertebral fractures [31, 32], but others have not found evidence of an association [33, 34]. A study among postmenopausal women in Beijing found no difference in prevalence of vertebral fracture between women with and without diabetes (OR = 1.04; 0.58–1.88) [35]. However, when women were stratified by BMI, diabetes was associated with higher vertebral fracture prevalence in the non-obese (BMI <25 kg/m2) women (OR = 2.79; 1.16–6.68).
Risk Factors for Fracture in T2D
Traditional risk factors for fracture include lower bone density, lower BMI, and increased frequency of falls. These factors are also associated with fracture in those with T2D [28, 30, 36].
Bone Density
As noted above, T2D presents a paradox of increased fracture risk in spite of higher bone density. A meta-analysis by Vestergaard reported higher BMD, measured by dual X-ray absorptiometry (DXA) and expressed as Z-score, at the lumbar spine and total hip in those with T2D [14]. Higher BMD was also found by Ma et al. in a more recent meta-analysis of age-adjusted results at the lumbar spine, total hip, and femoral neck [13]. BMD at the radius did not differ by diabetes status. BMI is positively associated with BMD in T2D [13] and broader populations [37] and may account for at least some of the higher BMD with T2D. However, when Ma et al. calculated pooled estimates using results with further adjustment for BMI and other factors, BMD remained higher at the hip (Fig. 2.2) and spine (Fig. 2.3) in T2D. All but one of the studies were conducted in Western countries, characterized by a high prevalence of obesity with T2D. Notably, the one study conducted in East Asia that was included in this meta-analysis found no statistically significant difference in BMD by diabetes status at the femoral neck or lumbar spine while BMD at the radius was lower with T2D [38]. Other studies in East Asian countries have found lower [39–41], similar [42–44] and higher [42–46] BMD at the hip and/or spine in those with diabetes. Two studies in China have assessed the relationship between DM and BMD, stratified by BMI. Among postmenopausal women in Shenyang, Zhou et al. reported lower BMD at the femoral neck and total hip with DM among non-obese (BMI <25 kg/m2) women [47]. Among obese women, BMD was higher but not statistically different between those with and without DM. However, the Peking Vertebral Fracture Study among women in Beijing found higher spine BMD with DM among the non-obese and no difference by diabetes status in the obese women [35]. A recent study in the United States, using high resolution pQCT rather than DXA, found evidence of reduced cortical bone density and thickness with greater cortical porosity, in non-obese T2D women compared with controls or with obese T2D women [48].
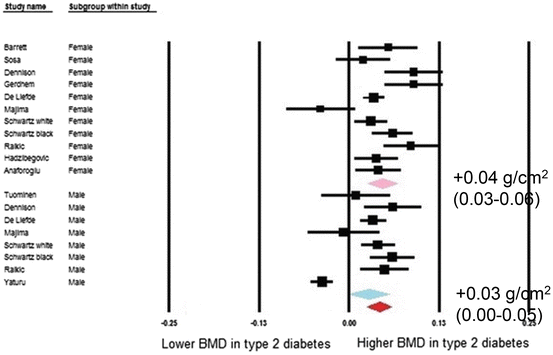
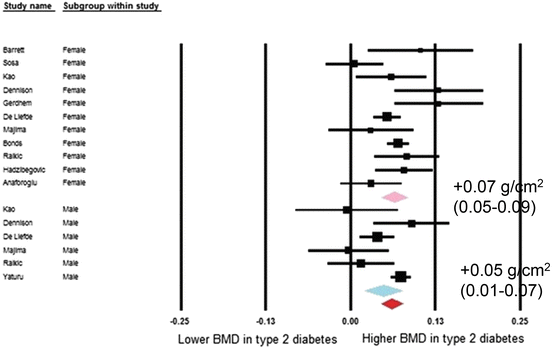

Fig. 2.2
Forest plot for mean femoral neck bone mineral density. Difference in means (g/cm2) and 95 % confidence interval for femoral neck bone mineral density between comparison groups with and without type 2 diabetes mellitus, stratified per study and gender. Diamonds represent joint estimate for subgroups of available studies for women (upper) and men (middle), respectively. Pooled estimate for all studies displayed with the diamond at the bottom [13]

Fig. 2.3
Forest plot for mean spine mineral density. Difference in means (g/cm2) and 95 % confidence interval for femoral neck bone mineral density between comparison groups with and without type 2 diabetes mellitus, stratified per study and gender. Diamonds represent joint estimate for subgroups of available studies for women (upper) and men (middle), respectively. Pooled estimate for all studies displayed with the diamond at the bottom [13]
A few studies have considered change in BMD in those with diabetes. The results appear to differ by skeletal site. Somewhat surprisingly, several have reported greater bone loss with T2D at the hip in spite of higher baseline BMD. In contrast, bone loss at the radius does not appear to differ with diabetes status. Results for spine BMD have been inconsistent. In the Study of Osteoporotic Fractures (SOF) among older white women, bone loss was more rapid in those with T2D at the total hip, femoral neck, spine, and calcaneus but was not different at the distal radius [49]. Greater bone loss at the femoral neck with diabetes was also reported among white women in a cohort study of older adults; bone loss did not differ by diabetes status in white or black men or black women [50]. In the placebo group of the Fracture Intervention Trial, women with diabetes had faster bone loss at the total hip [51]. In a longitudinal study of perimenopausal women, those with diabetes lost bone more rapidly at the total hip, but preserved bone relative to nondiabetic women at the spine [52]. Krakauer et al. reported no differences in bone loss at the radius by T2D status over 12.5 years of follow-up [53].
The relationship between bone density and fracture in T2D has been an important focus of research. Longitudinal studies have demonstrated that lower BMD is a risk factor for fracture in T2D as in broader populations [27, 54]. However, at any given BMD those with T2D have a higher risk of fracture than those without diabetes. This discrepancy has implications for fracture risk assessment, discussed in Chap. 3. It also implies that there are other factors contributing to fracture risk in T2D, beyond BMD. In broad terms, this additional fracture risk at a given BMD might be the result of increased frequency of falls or reduced bone quality that is not captured by BMD measurements, or both. Evidence that more frequent falls do not fully account for increased fracture risk with T2D (discussed in more detail below), combined with evidence from rodent models [55], has led to the conclusion that diabetic bone is more fragile for a given BMD. Understanding the aspects of bone that are affected by diabetes and that result in fragile bone has been an important focus of research on diabetes and skeletal health.
Body Size
Higher BMI is associated with lower risk of fracture in those with T2D [16, 36, 56]. A study of adults (40+ years old) with a DXA scan record in the Manitoba health registry made an explicit comparison of BMI as a risk factor for fracture in those with (n = 6455) and without (n = 55,958) diabetes [36]. BMI was higher in those with diabetes, but the relationship with fracture did not differ. In models adjusted for femoral neck BMD and other risk factors in the FRAX calculator, a 5 kg/m2 increase in BMI was associated with a lower rate of hip fracture in those with (HR = 0.81; 0.69–0.95) and without (HR = 0.82; 0.76–0.89) diabetes (p for interaction = 0.891). For the outcome of “major osteoporotic fracture” the relationship was also not statistically different (p for interaction = 0.080). If anything, the relationship was stronger in those with (HR = 0.90; 0.83–0.98) compared to those without (HR = 0.98; 0.95–1.02) diabetes.
Falls
About 90 % of fractures are due to a fall although less than 5 % of falls result in a fracture [57]. Falls are associated with fracture risk in those with T2D just as in broader populations [28, 58]. Those with T2D have a moderately increased risk of falling. A meta-analysis of eight studies estimated an increased risk of 1.19 (95 % CI 1.08–1.31), comparing those with and without diabetes [59]. However, insulin-treated patients appear to have a 2–3 times higher risk of falls compared with nondiabetic patients [60, 61]. In addition, several [62–64] although not all [65] studies of serious fall injuries resulting in a hospital or emergency room visit have reported higher incidence for T2D patients. These studies did not include information on hypoglycemia, discussed below.
This increased frequency of falls has been proposed as an explanation for the increased fracture risk observed among T2D for a given BMD. Importantly, as noted earlier, several studies of diabetes and fracture have included data on falls and have been able to address this question. In these observational studies, more frequent falls did not fully account for the increased risk of fracture observed with T2D [17, 33, 58, 66]. Higher fracture risk associated with T2D persisted even with adjustment for increased frequency of falls in these cohorts.
Diabetes-Related Risk Factors for Fracture
Investigations into diabetes-related risk factors for fracture have assessed the possible contributions of diabetes duration, presence of diabetes-related complications, glycemic control, and diabetes medications. Evidence that diabetes medications influence skeletal health is discussed in Chap. 7.
Diabetes Duration
Those with longer duration of diabetes have a higher risk of fracture [15, 16, 18, 19, 21, 33, 66–69]. For example, in a longitudinal cohort of Chinese in Singapore, the relative rate of hip fracture was 1.40 (1.08–1.82) in diabetic participants with duration of less than 5 years, compared with nondiabetic participants, and 2.66 (2.04–3.47) in diabetic participants with duration of 15+ years [21]. Longer duration of diabetes also appears to be associated with greater frequency of falls [70]. Reasons for this higher risk of fractures and falls with greater duration of diabetes may include increased frequency of insulin therapy and diabetes-related complications.
Glycemic Control
The effect of glycemic control on fracture risk, BMD, and falls remains poorly understood and controversial. On the one hand, reducing A1C levels is a standard goal of diabetes care that has been shown to reduce microvascular complications [71]. It is reasonable to assume that improved control might also have positive effects on bone health. On the other hand, lower A1C levels increase the frequency of hypoglycemic episodes which may increase the risk of falls and fractures. These hypotheses have been tested in a randomized trial, the Action to Control Cardiovascular Risk in Diabetes (ACCORD), a comparison of intensive and standard glycemic control in a cohort of patients with long-term T2D at increased risk of cardiovascular disease age 40–79 years [72]. The median achieved A1C in ACCORD was 6.4 % in the intensive and 7.5 % in the standard glycemic control groups [73]. In an ancillary study, ACCORD BONE, assessment of incident fractures and falls was added to the trial. This trial found no difference in fracture rates between the two treatment groups (HR = 1.04; 0.86–1.27) [74]. These results may have been influenced by the greater degree of TZD use in the intensive glycemic control group. As discussed in Chap. 7, fracture rates are doubled in women, but not men, using a TZD [75]. However, considering the fracture results only among men, whose fracture rates are less likely to be affected by TZD use, there was no evidence of a difference in fracture rates between the intensive and standard glycemic control groups (HR = 0.93; 0.70–1.25).
The trial also considered the effect of intensive versus standard glycemic control on falls. There were more hypoglycemic episodes in the intensive treatment group [73], but there was no overall effect of intensive control on the rate of falls (rate ratio = 1.10; 0.84–1.43). In analyses stratified by age, there was no evidence of increased falls with intensive control among those 65–79 years old (HR = 0.75; 0.55–1.01). In sum, the ACCORD trial provides evidence that reducing average A1C to 6.4 % does not reduce or increase fracture or fall risk compared with an average A1C of 7.5 % in older adults (40–79 years old). While the intensive treatment did not reduce fractures, it was also safe with regard to fractures and falls.
The ACCORD trial does not address the effects of poor glycemic control compared with standard (or intensive) control on fractures or falls. This question has been considered recently in several observational studies [76–79]. Three longitudinal studies have reported increased fracture risk with poor control. The largest study, using health registry data in Taiwan, included 1514 hip fracture cases in T2D patients [76]. Those with baseline A1C levels of 9–10 % (HR = 1.24; 1.02–1.49) and 10 % + (HR = 1.32; 1.09–1.58) had increased rates of hip fracture compared with patients whose A1C level was 6–7 %. There was a suggestion of a higher rate in those with A1C <6 % (HR = 1.19; 0.97–1.45) compared with 6–7 %, but the difference was not statistically significant. Those with a baseline A1C of 7–8 % had a slight increase in hip fracture rate (HR = 1.07; 0.92–1.25) compared with 6–7 %. This result is generally consistent with the report from the ACCORD BONE trial comparing fracture rates in men in the standard (median A1C 7.5 %) and intensive (median A1C 6.4 %) control groups (HR = 1.08; 0.80–1.43) [74].
In the Rotterdam cohort of older adults, baseline measurements of fructosamine were converted to A1C equivalent units; the median A1C was 7.5 % among those with type 2 diabetes [77]. Diabetic participants with A1C ≥7.5 % had a higher rate of fracture, in spite of higher BMD, compared with diabetic participants with A1C <7.5 % (HR = 1.54; 95 % CI 1.04–2.29, adjusted for age, sex, height, and weight). The fracture incidence was 31.1 per 1000 person-year in those with A1C ≥7.5 % and 23.0 per 1000 person-year for those with A1C <7.5 %, an absolute difference in fracture rates of 8 per 1000 person-years. A second longitudinal study, based on the Atherosclerosis Risk in Communities (ARIC) Study, compared rates of fractures identified through hospitalizations [78]. Among those with diagnosed diabetes, the average A1C was 8.3 (SD 2.3). Those with A1C ≥8 % had a higher rate of fracture than those with lower A1C (HR = 1.63; 95 % CI 1.09–2.44, adjusted for age, sex, race, BMI, and other factors). Ten-year cumulative incidence of hospitalized fracture was 4.9 % in those with A1C ≥8 % and 4.4 % in those with A1C <8 %.
In contrast to these three studies that considered long-term effects of poor glycemic control on fracture risk, Puar et al. considered a somewhat different question, analyzing hip fracture risk within 3 months after assessment of A1C, among diabetic patients admitted to Changi General Hospital in Singapore over a 5-year period [79]. In this analysis of short-term effects of glycemic control, lower A1C in the previous 3 months was associated with reduced hip fracture risk. Compared with the reference group (A1C >8 %), diabetic patients with A1C <6 % (OR = 3.0; 2.0–4.5), A1C between 6.1 and 7 % (OR = 2.4; 95 % 1.7–3.2), or A1C between 7.1 and 8.0 % (OR = 1.2; 95 % CI 0.8–1.6) had higher odds of a hip fracture. In contrast, the ACCORD trial did not find increased fracture risk with sustained intensive glycemic control (median A1C 6.4 %) compared with standard control (median A1C 7.5 %) [74].
Studies of the effect of A1C on falls have yielded inconsistent results. In a study of incident falls among older white and African-American adults in the United States, there was no association between glycemic control and falls for those using an oral antidiabetes medication [80]. However, among those using insulin, low baseline A1C (≤6 %) was associated with increased risk of falling. A study of US adults 75 years of age and older also found increased risk of falls with lower A1C (≤7 %). In contrast, a study in London reported increased falls in older adults (65+ years) in those with poor glycemic control (A1C >8 %) [81]. A study in older African-American adults in the United States found no association between glycemic control, assessed with fructosamine, and falls [82]. In a study that was limited to falls resulting in an injury requiring hospitalization, poor glycemic control (A1C of 8 % or higher) was associated with increased risk among older adults with T2D [64].
The most appropriate A1C goal for treatment of diabetes in older adults remains controversial [71, 83, 84]. There are unanswered questions regarding the net benefit of a lower target in this age group, and the effects on falls and fractures are an important part of this equation. The ACCORD trial suggests that an A1C target of 6.4 % is safe with regards to falls and fractures but notably the trial did not include any participants over age 79 years. ACCORD also indicates that prevention of fractures and falls is not a motivation for maintaining A1C levels below 7.5 %. Observational studies to date are limited but suggest that an A1C target of less than 8 % could reduce fractures.
Hypoglycemia
Severe hypoglycemia is relatively common in T2D [85, 86]. A recent survey in a California HMO found that about 10 % of T2D patients experience an episode of severe hypoglycemia during a year [86]. Severe episodes were more prevalent among those with near normal glycemia and those with poor control (A1C ≥9 %). It is believed that hypoglycemic episodes lead to falls and increase the risk of fractures. However, there has been surprisingly little study of these associations. A study using Danish health registry data found higher fracture risk associated with a prior episode of hypoglycemia (HR = 1.13; 1.00–1.26) [87]. In a study designed to assess the relationship between hypoglycemic events and fracture, using a healthcare claims database in the United States, T2D patients with a hypoglycemic event requiring medical care during a 1-year period of observation had an increased risk of a fall-related fracture during the same year (adjusted OR 1.70; 95 % CI 1.58–1.83) [88].
Diabetes-Related Complications
Diabetes duration may increase fracture risk in part through an increase in the prevalence of microvascular and macrovascular diabetic complications. Certainly in broader populations there is evidence that the manifestations of microvascular complications, particularly reduced vision [89] and kidney disease [90], contribute to fracture risk. Similarly, the manifestations of macrovascular complications increase fracture risk in broader populations, including stroke [91], myocardial infarction [92] and peripheral arterial disease [93].
Diabetic patients with multiple complications appear to be at higher risk of fracture, but results are mixed for the association between specific complications and fracture. A study using Danish registry data found increased risk of any fracture in T2 diabetic patients with eye disease (OR = 2.1; 95 % CI 1.8–2.4), kidney disease (RO = 2.0; 1.6–2.5), diabetic neuropathy (1.9; 1.6–2.2), or macrovascular complications (OR = 1.9; 95 % CI 1.6–2.3) while T2 diabetes without complications was associated with a more modest increase in fracture risk (OR = 1.4; 95 % CI 1.4–1.5), all compared with nondiabetic patients in unadjusted models [94]. However, after multiple adjustment, including for the presence of other complications, these estimates were attenuated. Those with multiple complications had an increased risk of fracture (adjusted OR = 1.3; 95 % CI 1.2–1.5) as did those with uncomplicated T2 diabetes (adjusted OR = 1.13; 95 % CI 1.06–1.22), but specific complications were not associated with fracture risk in adjusted models. A population-based study using medical records in Rochester, Minnesota, assessed diabetic complications as risk factors for fracture in models limited to those with T2D [16]. Increased fracture risk was reported for neuropathy (age-adjusted HR 1.4; 95 % CI 1.2–1.7) but not for clinically diagnosed nephropathy (age-adjusted HR 1.1; 0.8–1.3) or retinopathy (age-adjusted HR 1.0; 0.8–1.2). However, renal failure was associated with higher fracture risk (age-adjusted HR = 1.6; 1.2–2.2).
A study using fundus photography to identify retinopathy, conducted as part of the Blue Mountains Eye Study in Australia, found increased fracture risk with diabetic retinopathy [68]. In a US study with direct measurements of monofilament detection and serum creatinine, Strotmeyer et al. reported higher fracture risk among diabetic participants with inability to detect 10 g monofilament, but no increased risk associated with high creatinine [58]. A study of risk factors for prevalent vertebral fractures among T2D women in Brazil found increased prevalence of fractures in those with diabetic retinopathy, identified by funduscopy, and those with lower creatinine clearance, but no difference in fracture prevalence based on clinical nephropathy or peripheral neuropathy [95].
For macrovascular complications, history of stroke is associated with higher fracture risk in older adults with T2D [58]. A mediation analysis, based on data from the Cardiovascular Health Study, found that ankle-arm index, a measure of peripheral arterial disease, accounted for a substantial portion of the higher hip fracture risk associated with diabetes [56].
Prediabetes and Fracture Risk
Those with glucose levels below the threshold defining diabetes but above normal levels have a higher risk of developing diabetes. Primarily on this basis, a category of “prediabetes” has been defined using fasting glucose, the oral glucose tolerance test, or A1C levels [71]. Prediabetes predicts the development of cardiovascular disease as well as the development of diabetes [96]. The effect of prediabetes on fracture risk has been assessed in a limited number of studies with inconsistent results. None of the studies have reported a statistically significant increased risk of fracture with prediabetes although power in each study was limited. Some have reported lower risk of fracture among those with elevated 2 h oral glucose tolerance test (OGTT) results, but elevated fasting glucose (FG) has not been associated with reduced risk of fracture. In the Rotterdam study, participants with impaired glucose tolerance based on OGTT (7.8–11.1 mmol/L) had a lower risk of non-vertebral fracture compared with nondiabetic participants (RR = 0.80; 0.63–1.00) in multivariable models including BMD [66]. The Malmo Preventive Project also found evidence of lower osteoporotic fracture risk among those without diabetes in the highest quartile of OGTT but not among those with elevated fasting glucose (FG) [97]. The AusDiab study reported a similar pattern in women, but not men. Nondiabetic women in the highest quartile of OGTT had a reduced risk of a low-trauma fracture (age and BMI-adjusted OR = 0.59; 0.40–0.88) but fracture risk did not differ across levels of FG [98]. For men, FG and OGTT (age and BMI-adjusted OR = 1.39; 0.60–3.26, comparing upper and lower quartiles of OGTT) were not associated with fracture risk. The Osteoporotic Fractures in Men (MrOS) study in the United States also found no association between prediabetes, defined by FG, and risk of non-vertebral fracture (age and BMD-adjusted HR = 1.04; 0.89–1.21) [28]. In contrast, in older US adults, fracture risk in those with impaired fasting glucose (110–125 mg/dL) was modestly elevated but not statistically different compared with participants who had normal fasting glucose in multivariable models including BMD (HR = 1.34; 0.67–2.67) [58]. A larger US study based on NHANES data similarly reported modestly elevated, but not statistically different, risk of any fracture in those with prediabetes, defined by A1C (5.7–6.4 %), compared with no diabetes (A1C <5.7 %) in multivariable models including BMI [29]. The adjusted HR for any fracture was 1.20 (95 % CI 0.96–1.51) in non-Hispanic whites and 1.42 (95 % CI 0.72–2.81) in Mexican-Americans.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree