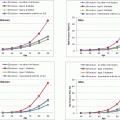
Fig. 6.1
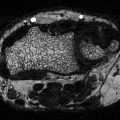
Histomorphometric changes in bone formation. (a, b) Tetracycline double-labeled bone biopsies in a 58-year-old T2D Caucasian woman (a) and a 57-year-old Caucasian female control (b). Bone formation is decreased in T2D with reduced mineralizing surface. The arrows highlight tetracycline uptake in the control subject and the absence of uptake in the diabetic subject. Adapted with permission from [46]
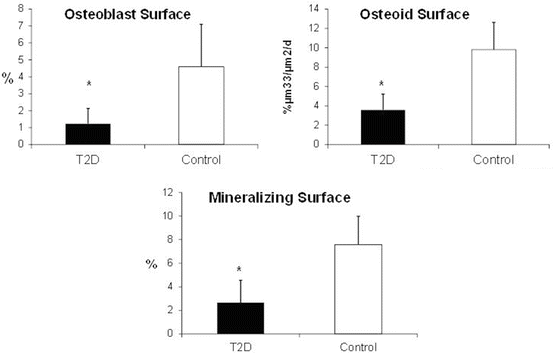
Fig. 6.2
Quantitative measures of bone formation were lower in T2D postmenopausal women than in controls. Adapted with permission from [46]
PTH
Levels of PTH, a key regulator of bone remodeling, are altered with glycemia and diabetes. In healthy subjects, a glucose load leads to a slight decrease in ionized calcium and an increase in PTH, after an initial temporary decrease [28]. In T1D, blunted PTH responses have been observed [47]. In T2D, levels of PTH tend to be 20–50 % lower than in controls, even in the setting of reduced eGFR, suggesting a state of reduced PTH secretion [39, 40, 43]. The importance of PTH in diabetic bone disease has been corroborated by the beneficial skeletal effects of PTH treatment in T2D rats [48]. Specifically, PTH treatment partially reversed the adverse skeletal effects of T2D on bone mass, bone strength, and bone defect repair [48].
Nonclassical Bone Biomarkers
IGF-1, an anabolic factor which stimulates osteoblast proliferation, has been inversely associated with the risk and number of vertebral fractures in T2D postmenopausal women independent of BMD [40, 49]. Circulating osteogenic precursor (COP) cells [50] might also be a biomarker for diabetic bone disease. COP cells can be detected in the peripheral blood by flow cytometry using antibodies specific for the osteoblast matrix protein osteocalcin (OCN) [50, 51] or alkaline phosphatase [52] and are capable of mineralization in vitro [52]. Certain subpopulations of OCN+ cells (OCN+/CD133+/CD34−/KDR+, known as calcifying cells) were increased in subjects with an HbA1c in the prediabetic range [53], although in another report, peripheral blood mononuclear cells that were positive for osteocalcin were lower in postmenopausal women with T2D as compared to nondiabetic controls [46]. An additional potential biomarker of diabetic bone disease is RANKL. Kiechl et al. showed that increased levels of soluble RANKL were associated with the development of diabetes in 844 subjects (OR = 3.37; 95 % CI: 1.63–6.97) [54]. Another novel bone marker in T2D may be sphingosine 1-phosphate (S1P), a lipid mediator which increases osteoclastogenesis by increasing RANKL [55]. S1P was found to be increased in T2D women (n = 482) as compared to controls and was associated with increasing numbers of vertebral fractures and bone resorption markers [55]. Interestingly, this marker suggests an elevation in bone resorption in T2D, in contrast to the reports of reduced biochemical markers of bone resorption [39–41, 43].
Sclerostin
Sclerostin, an osteocyte product, is a negative regulator of bone formation which competes with the anabolic Wnt b-catenin pathway by binding to LRP5 or 6 [56]. In healthy adults, sclerostin levels are increased by factors including age, BMI, inactivity, bone mineral content, and possibly fractures [56]. It was first reported in 2012 that sclerostin levels were higher in 74 T2D women and men vs. 50 nondiabetic controls, and that higher levels correlated with age, male gender, and BMD [57]. In a different study which included T1D (n = 43), T2D (n = 40) and matched controls, sclerostin levels did not differ between T1D subjects and controls, but were twofold higher in T2D than in controls or T1D, even after adjusting for age and BMI [58]. These data suggest that the Wnt signaling pathway may be impaired in T2D, although no relationship between markers of bone formation and sclerostin was found [58]. Interestingly, T2D subjects also had lower PTH levels [58], raising the possibility that since PTH inhibits sclerostin [59], perhaps the higher sclerostin levels were due to a decrease in the usual inhibitory effect of PTH. A correlation between Wnt disruption and decreased osteoblast activity was further observed in 40 T2D postmenopausal women who, as compared to controls, had decreased β-catenin levels which correlated with lower BAP [41]. In the largest diabetes sclerostin study, higher sclerostin levels in 321 men and women with T2D were associated with an increased risk of vertebral fractures independent of lumbar spine BMD [60]. It could be posited from these data that because sclerostin is secreted from deeply embedded osteocytes that are in mature bone tissue, the higher sclerostin levels reflect an increase in the amount of aged bone mass that is more likely to accumulate microfractures independent of BMD. Further work will be needed to clarify this point. In a rat model of T2D, treatment with an anti-sclerostin antibody enhanced bone mass and reversed femoral defects [61], although this model did not fully replicate adult-onset diabetes because diabetes developed before skeletal maturity.
Advanced Glycation End Products
Increased levels of advanced glycation end products (AGEs) have been shown to be biomarkers for diabetic bone disease. In the setting of chronic hyperglycemia, AGEs accumulate in the organic bone matrix by a process known as nonenzymatic glycation (the Maillard reaction) [62–69]. Accumulation of AGEs in the organic bone matrix leads to more biomechanically brittle bone that has lost its toughness and is less able to deform before fracturing [63]. Circulating levels of pentosidine, one of the best studied AGEs, have been shown to correlate with bone AGE levels [70]. In 104 nondiabetic patients undergoing orthopedic surgery, plasma pentosidine levels correlated with cortical bone pentosidine [70]. In a nondiabetic population of 765 postmenopausal women, an increase in urinary pentosidine levels predicted a 20 % increase in vertebral and long bone fractures over 5 years [71]. However, pentosidine levels do not consistently distinguish diabetics and nondiabetics when measured in urine [72] or serum [73]. Nevertheless, pentosidine levels appear to provide information about diabetic bone fragility that is separate from BMD parameters. In 1000 patients followed for 7.5 years, urinary pentosidine levels in those with diabetes were associated with a 42 % increase in clinical fracture incidence [relative hazard, 1.42; 95 % confidence interval (CI), 1.10, 1.83] and a nearly sixfold increase in vertebral fracture prevalence, independently of BMD [72]. Similarly, in 153 Japanese men and women with T2D, serum pentosidine levels were significantly higher in the women who had vertebral fractures, independent of BMD (OR 2.50, CI: 1.09–5.73) [73]. With regard to direct measurement of pentosidine in diabetic bone, preliminary data in T1D patients showed that iliac crest bone biopsies in T1D who had fractured had higher pentosidine levels than controls, in association with a greater degree of mineralization [74]. In addition to pentosidine, circulating levels of another AGE, carboxy-methyl-lysine (CML), might indicate bone fragility. When 3373 nondiabetic patients were followed for 9 years, an increase in CML predicted a 27 % increase in hip fracture risk, independent of hip BMD [75].
Architectural Properties in T2D
In addition to alterations in remodeling and matrix properties, another factor that may contribute to the paradox of increased fractures despite normal areal BMD in T2D is microarchitectural abnormality. Increased cortical porosity, a key determinant of bone fragility [76], has been reported at the radius and tibia in female diabetics who have fractured, as measured by intra-cortical pore volume fraction via high-resolution peripheral quantitative computed tomography (HR-pQCT) [77]. In a recent community-based study of women and men, T2D and increased HbA1c levels were associated with deficits in cortical microstructure and density at the distal tibia [78]. Microarchitectural deficits in bone geometry might also explain reduced bone strength in T2D. Strength-to-load ratios (QCT) at the spine and femoral neck were not improved in older adults with T2D although areal BMD (DXA) was higher [79]. In a study of older men, volumetric BMD (pQCT) was higher but bone area was smaller at the distal radius and tibia [80]. Smaller cross-sectional area suggests that stimulation of periosteal apposition, normally observed with greater loading, may be reduced in diabetes. These data seem to suggest that the higher areal BMD in diabetics does not result in the expected biomechanical advantage and supports the likelihood of abnormalities in dynamic and material properties.
Conclusions
At a time when classical complications of diabetes mellitus are becoming less common due to improved glucose control, the skeleton has emerged as a target organ for disease complications. It is now well established that fracture risk is increased in both T1D and T2D. Yet BMD and FRAX, the clinical mainstays for predicting fractures, do not fully capture fracture risk in these populations. Identification of biomarkers in diabetic individuals that predict fracture risk, independent of bone density assessment, will help to diagnose and treat bone fragility in diabetes. Use of biomarkers in diabetes will hopefully offset serious skeletal challenges in this population as they age.
Acknowledgments
Funding Source: Columbia University Irving Institute for Clinical and Translational Research CTSA/CTO pilot award
Disclosure: The author has nothing to declare
References
1.
IDF diabetes atlas update. 2012. http://www.idf.org/diabetesatlas/5e/Update2012. Accessed 10 Jan 2013.
2.
3.
4.
Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–92.PubMedCentralCrossRefPubMed
5.
6.
7.
8.
9.
Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012;83(6):653–60.PubMedCentralCrossRefPubMed
10.
11.
12.
13.
14.
15.