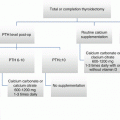
Fig. 21.1
Whole-body Iodine scan (I-123) showing extensive radioiodine uptake in the left proximal humerus. Additional metastatic foci are present in the right iliac crest, proximal left femur, the right 5th and 9th ribs, and other multiple skeletal regions
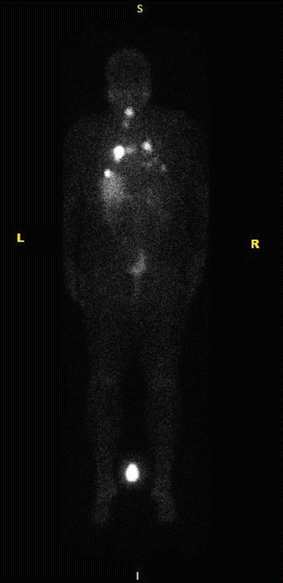
Fig. 21.2
Whole-body iodine scan (I-123) showing focal uptake of iodine-avid disease over the lower neck and lungs
FDG/PET is usually performed in the setting of elevated Tg with a negative WBS [4, 26]. This imaging modality can offer additional information in about 10 % of these patients [26]. A review of the performance of FDG/PET for the diagnosis of recurrent/metastatic thyroid cancer showed variable sensitivity (45–100 %) but good specificity (90–100 %). Variables that can explain these wide ranges include tumor burden, previous imaging studies before the FDG/PET, and location of metastatic disease [27]. The rate of cases in which the results of FDG/PET changes clinical management is also quite variable with values between 9 and 54 %. The exact advantage of FGD/PET when compared to other diagnostic modalities and the most cost-effective diagnostic algorithm for recurrent or metastatic disease including FDG/PET is not known at this time [26, 27].
Indications for Radioactive Iodine in Differentiated Thyroid Cancer
The use of RAI in the treatment of differentiated thyroid cancer is reserved for clinical situations in which its benefits outweigh the potential risks and burden associated with treatment. RAI use should be considered in the following clinical situations: (i) remnant ablation after thyroidectomy, (ii) as adjuvant therapy for micrometastases, (iii) local recurrent disease not amenable to surgery, and (iv) treatment of metastatic disease [4]. The first two indications are covered elsewhere in this book (see Chap. 23). In this chapter, we will focus on the treatment of recurrent and metastatic disease.
Rationale for Therapy
Recurrent disease after initial surgical treatment of DTC can occur, locally in the anterior neck or cervical lymph nodes or in distant sites, most commonly lungs and bones, although rarely other less frequent sites such as kidney, liver, and brain can be affected [4, 8]. Most recurrences occur in the lymph nodes during the first few years of follow-up, suggesting that lymph node involvement could represent persistent (rather than recurrent) disease, followed by subsequent enlargement [4, 8, 28]. The difference between true local recurrence and lymph node metastases is important from a surgical perspective, since local recurrence is defined as newly formed tumor within the soft tissue, in comparison with well-defined disease found in lymph nodes [28].
The treatment options for recurrent disease include observation, RAI, reoperation, and radiation therapy [4, 28]. Percutaneous ethanol ablation has also been used successfully in selected centers with experience in this procedure, for treatment of limited locoregional recurrences involving nodules in the thyroid bed or lymph nodes in the central or lateral neck [29, 30]. The use of RAI is usually reserved for cases in which treatment is needed but surgery is not advisable/feasible [4, 31].
There seems to be an association between recurrent local disease and patient outcomes such as mortality. A large study of almost 6000 patients found rates of recurrence during follow-up of 7 % for local lymph nodes, 2 % for lung, and 0.6 % for bone metastases, in addition, 1 % of the patients died from causes related to DTC [32]. The strongest predictors for death related to thyroid carcinoma were age older than 55 years, extra nodal tumor extension, and large nodal metastases. The authors suggested that recurrent lesions in older patients with evidence of extra nodal extension can represent life-threatening disease and should be aggressively managed [32]. Another study that evaluated 201 patients with DTC also found an association between the presence of locoregional recurrence and distant metastases with increased mortality on univariate analysis, but these finding remained statistically significant only for distant metastases on multivariate analyses [33].
In a study of approximately 1000 patients with DTC, 42 were found to have lung metastases during follow-up using iodine WBS and were treated with RAI with stabilization or resolution of tumor noted in 7 and 10 patients, respectively. The mean cumulative I131 activity was 410 ± 240 mCI [34]. Another study evaluated 101 patients with lung metastases from thyroid cancer, noting that the 5- and 10-year survival rate was higher in those with positive iodine uptake compared with those without uptake [35]. Another study that evaluated 444 patients with metastatic disease from DTC, including 223 with lung metastases, 114 with bone disease, and 82 with both bone and lung disease, found 10-year survival of 92 % in those patients who responded to therapy compared to 19 % without treatment response. Patients in this cohort were treated with 100 mCi of I131 after thyroid hormone withdrawal every 3–9 months for 2 years and then yearly until response (defined as negative scan) was achieved. Negative imaging studies were achieved in 43 % of the patients, most commonly in younger patients, with well-differentiated tumors and limited extent of disease. The negative studies were achieved after cumulative doses of 100–600 mCi. In the case of bone metastases, only 17 % achieved remission in comparison to 74 % of those with lung disease [36].
Preparation for Radioactive Iodine Treatment
To enhance the effectiveness of RAI therapy (its uptake and retention by malignant thyroid follicular cells), two strategies are commonly utilized: (1) depleting iodine stores and (2) increasing serum TSH levels [4, 12].
Low Iodine Diet
Current treatment guidelines recommend restriction on dietary iodine before RAI with the goal of achieving depletion of the body iodine stores and increasing RAI uptake by thyroid cells [4]. This recommendation is supported by a systematic review and meta-analysis that found that a low iodine diet decreases urinary levels of iodine, increases RAI uptake, and can possibly increase the efficacy of treatment. The most common approach is to limit dietary iodine to ≤50 mcg/day for 1–2 weeks [37]. During that period, avoidance of iodized salt (non-iodized salt may be used instead) and other foods rich in iodine, such as kelp, seaweed, and shellfish, is recommended. A study that evaluated 125 patients who followed a restrictive iodine diet found that 15 days were sufficient to achieve iodine depletion when measured both by a 24-hours and by a spot urinary iodine measurement [38].
However, there is conflicting data from other centers where dietary iodine restriction has not been associated with significant clinical benefits [39].
Increasing TSH
An arbitrary level of TSH stimulation of more than 30 IU/L is generally used as the cutoff above which adequate I131 uptake by the thyroid cells is expected to result in increased effectiveness of RAI therapy. Two main approaches are currently used to achieve thyroid stimulation: (1) thyroid hormone withdrawal (THW) and (2) recombinant human TSH stimulation (rhTSH) [3, 4, 40].
Many methods on how to perform THW have been described. These include (a) discontinuation of levothyroxine therapy 3 weeks before RAI treatment and (b) reducing the levothyroxine dose for 3 weeks followed by its discontinuation and replacement with T3 therapy (shorter half-life) for 2–4 weeks, which is then discontinued 2 weeks before RAI treatment. There does not appear to be a major difference in terms of symptoms of hypothyroidism or time to TSH elevation to necessarily prefer one method over the other. The main disadvantage of these withdrawal methods is the expected symptoms of hypothyroidism [3, 41–43].
Alternatively, rhTSH is often used to stimulate the iodine uptake to achieve ablation. However, although rhTSH has been approved for diagnostic purposes and for thyroid remnant ablation (see Chap. 23), it has not been approved by the US Food and Drug Administration for treatment of metastatic disease [12]. When RAI is used for remnant ablation, levothyroxine is continued and rhTSH is injected daily for 2–3 days before therapy. The main advantage of this approach is the avoidance of symptoms of hypothyroidism; the main disadvantage is its high cost. In addition, long-term studies regarding safety and efficacy outcomes in patients with metastatic disease are scarce [3]. However, some experience has accumulated from small studies in which this approach was used on a compassionate basis in patients unable to tolerate THW or mount an appropriate TSH response after withdrawal or in whom THW was contraindicated for medical reasons, showing that the use of rhTSH appeared to be safe and resulted in clinical improvement or stabilization of tumor burden in the majority of patients [44, 45]. Current ATA guidelines indicate there is insufficient evidence to recommend this therapy in all patients with distant metastatic disease, but allow for consideration of the use of rhTSH for treatment of metastases in selected patients in which iatrogenic hypothyroidism might be considered risky (e.g., patients with multiple comorbidities) and those with pituitary disease in whom TSH cannot be raised [4, 44, 45].
There are no randomized controlled trials evaluating the effect of rhTSH vs THW on safety and efficacy outcomes of the treatment of metastatic or recurrent disease. A review of observational studies found that patients prepared with rhTSH had positive post-therapy scans in 75 % of the cases and achieved complete or partial disease remission in 65 % of the cases [46]. In addition, observational studies comparing these preparation strategies are available, suggesting similar outcomes between these modalities. For example, an observational study that evaluated 84 patients with well DTC and RAI-avid lesions mostly in the regional neck (64 with rhTSH and 20 with THW) found no statistically significant difference between the rates of successful therapy in patients with RAI-avid metastatic disease prepared with one approach or the other [47]. In the study by Klubo-Gwiezdzinska et al., 56 patients with RAI-avid distant metastases treated with one of these approaches were followed for 72 months, and after adjusting for confounders, no difference in rates of clinical response or progression-free survival was found [48]. Another study of patients with metastatic thyroid cancer found no difference at 5.5 years of follow-up in terms of overall survival between patients prepared with rhTSH stimulation, THW, or THW followed by rhTSH stimulation [49].
The use of one approach over the other will then depend on the patient’s characteristics (age, comorbidities, and potential complications in case of hypothyroidism) and other system variables (e.g., costs).
RAI Dosing
The selection of the dose to use in cases of local or metastatic disease in patients with DTC can be determined using an empiric approach, measuring the upper bound limit of blood and body dosimetry, or by quantitative tumor dosimetry [4, 12]. There are no randomized controlled trials evaluating efficacy and safety outcomes between these various approaches or comparing different radiation doses. Using the empiric approach, doses of 100–150 mCi are used for local disease (with higher doses used in cases of extrathyroidal invasion). In the case of pulmonary metastases, doses of 150–200 mCi are used (lower doses in patients with very high uptake, due to risk of lung injury). These doses can be repeated every 6–12 months as long as there is evidence of RAI avidity [4, 12].
Alternatively, dosimetry can be used to select the treatment dose based on calculations that maximize the dose delivered to the tumor lesions, while limiting whole-body retention to 80 mCi at 48 h, or by calculating the maximum tolerated activity to the bone marrow (estimated at 200 cGy) [4, 12]. The dosimetry approach is currently practiced in only a limited number of institutions, despite evidence suggesting possible benefits. For example, in a study of 535 patients in which the majority had normal renal function, an empiric dose of 200 mCI would exceed the maximum tolerated activity in 8–15 % of patients < 70 years and in 22–38 % of patients 70 years or older [50]. Another analysis of 127 dosimetry studies found that using empiric doses between 100 and 300 mCi, the proportion of patients that would exceed the maximum tolerated activity ranged from 1 to 22 %, while 78–98 % could have received a higher dose, suggesting that dosimetry would be helpful in clinical practice [51].
Value of RAI Therapy in Patients with Non-RAI-Avid Structural Disease
Empiric RAI therapy of patients with elevated Tg and known or suspected structural disease, but negative iodine WBS, is controversial. There have been no randomized controlled trials assessing the benefit of this treatment in such patients. A systematic review of 13 observational studies assessing the efficacy of RAI treatment in patients with elevated serum Tg levels but negative iodine WBS found that Tg levels decreased after therapy in 62 % of the cases and positive post-therapy scans were seen in 56 % of the patients, suggesting a possible benefit [52]. However, 44 % of the patients included in five studies had reduction of Tg levels without treatment during follow-up. In two of these studies, no patient showed a decreased or normalized Tg during follow-up. Rigorous comparison between patients treated with one approach and the other is required to provide more reliable conclusions regarding the benefits of RAI in these patients [52]. One study compared 42 patients with positive serum Tg and negative diagnostic scan with 28 patients with similar characteristics but who were not treated. In the treated group, 70 % of patients had a positive post-therapy scan. Normalization of the serum Tg or resolution of the initial iodine uptake during follow-up was seen in 56 % of the cases showing lung uptake at the first post-therapy scan, and in 60 % of the cases showing cervical node metastases at the first post-therapy scan. In the untreated group, 70 % of patients had Tg levels that became undetectable during follow-up [53]. Current treatment recommendations suggest observation in patients without structurally evident disease and low Tg levels (Tg < 10 ng/dL with thyroid hormone withdrawal or < 5 ng/mL with rhTSH). Empiric therapy with RAI should be reserved for those with significantly elevated Tg (or rapidly rising levels) without a structural target amenable to directed therapy (surgery, alcohol ablation, external radiation, etc.) [4].
Post-therapy Scan
The utility of performing a post-therapy scan in patients who received therapy with RAI is also controversial; however, current ATA guidelines recommend obtaining a post-therapy scan [4]. A study of 143 I131 scans in 93 patients with DTC performed 5–12 days after RAI therapy found discordance between the scans in 22 % of the cases, with detection of new areas of uptake in 9 % of the cases (lung and cervical lymph node uptake). In addition, 12 % of the cases show increased areas of uptake in locations already diagnosed by the pretreatment scan. The authors concluded that in the majority of the cases, the new areas of uptake do not change clinical management; however, the post-therapy scan can help confirm the uptake of the iodine treatment and identify more clearly cervical lymph node disease [54]. Another study evaluated the concordance between pre- and post-therapy scans in 177 pairs and found concordance in up to 94 %. In 11 pairs of scans (6 %), the new focus of disease was found in the thyroid (6 pairs), cervical lymph nodes (3), lung (1), and bone (1). The new findings would have changed the clinical management in only 2 pairs (1 %) of scans (new lung and bone disease)[55].
Complications and Side Effects of RAI Therapy
The decision to utilize RAI for the treatment of DTC should be based on an evaluation of the benefits associated with treatment as well as risks. RAI therapy is associated with both acute and chronic side effects [56, 57].
The administration of RAI is generally well tolerated. Nausea and vomiting may occur in the acute setting in 50–70 % of the patients. These symptoms usually occur within a few hours of RAI administration and resolve by 1–2 days [57].
Another important side effect is sialadenitis that can lead to xerostomia, in up to 30 % of adults. This is thought to be due to the ability of the salivary glands to concentrate significant amount of iodine from the plasma [56]. Symptoms suggestive of salivary gland dysfunction are reported in approximately 60 % of patients treated with RAI, with most cases occurring within the first 6 months; the overall incidence of these symptoms increases as the RAI dose increases [56, 58].
A systematic review of two large observational studies found an increased relative risk for secondary malignancies and leukemia in patients treated with RAI compared to those that were not; the risk for other specific cancers was not elevated [59].
Conclusions
RAI is an effective treatment option for patients with DTC who are found to have recurrent or metastatic disease during follow-up. Patients who will undergo treatment with RAI should be placed on a low iodine diet (to deplete iodine stores) and have elevated TSH levels at the time of treatment (achieved with rhTSH or THW). The dose of RAI to be given can be based on empiric regimen strategies or estimated with the use of dosimetry. Patients should be counseled about the potential acute and chronic side effects and treatment burden of RAI.
References
1.
Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–22. doi:10.1001/jamaoto.2014.1.CrossRefPubMed
2.
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi:10.1155/2013/965212.CrossRefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree