Fig. 3.1
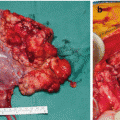
CT scan in a patient with urachal cancer through the upper abdomen showing large volume disease in the right upper quadrant, left upper quadrant, and lesser omentum (From Sugarbaker [59])
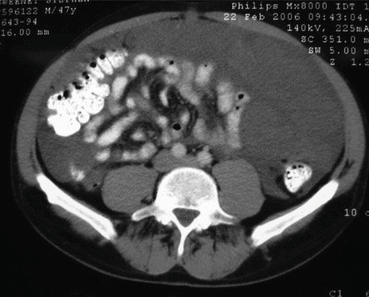
Fig. 3.2
CT scan in a patient with urachal cancer through mid-abdomen showing the “omental cake” characteristic of pseudomyxoma peritonei. The small bowel is compartmentalized beneath the omental cake (From Sugarbaker [59], with permission)
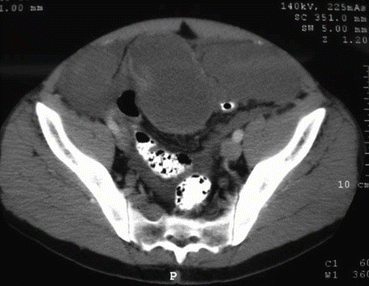
Fig. 3.3
CT scan in a patient with urachal cancer through the lower abdomen showing a cystic mass directly above the bladder (From Sugarbaker [59], with permission)
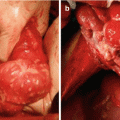
The patient was taken to the operating room in February 2006. At the time of surgery, the primary tumor was a supravesical mass that communicated with the bladder through a patent urachus and could be dissected clear of the bladder with a negative margin. Large volumes of intravesical mucus were suctioned through the opening of the dome of the bladder. No other bladder abnormalities were identified. The appendix, dissected free of the surrounding tissue, was determined to be normal. The findings at surgery showed extensive tumor infiltrating the omentum, beneath hemidiaphragms, and in the pelvis. The small bowel was free of disease (Fig. 3.4).
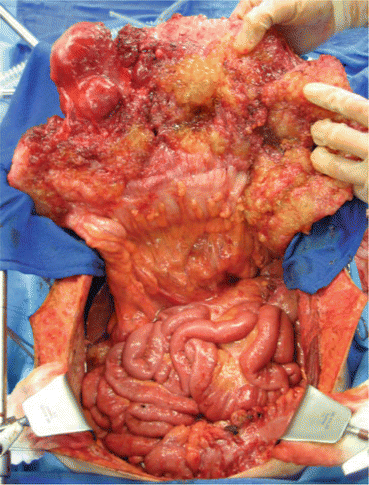

Fig. 3.4
Intraoperative photograph in a patient with urachal cancer showing the omental cake with normal-appearing small bowel beneath (From Sugarbaker [59], with permission)
Cytoreductive surgery required total anterior parietal peritonectomy, right upper quadrant peritonectomy, left upper quadrant peritonectomy, greater and lesser omentectomy, right colectomy, and pelvic peritonectomy including a rectosigmoid colectomy [18, 19]. Segmental cystectomy and closure of the bladder were performed. The tumor, widely distributed within the abdomen and pelvis, was reduced to no visible evidence of disease by CRS. A diverting ileostomy was performed to protect a low colorectal anastomosis. The patient received hyperthermic intraperitoneal mitomycin C (15 mg/m2) and doxorubicin (15 mg/m2) and simultaneous bolus intravenous 5-fluorouracil at 600 mg/m2 and leucovorin at 20 mg/m2 in the operating room. Tubes and drains were positioned so that patient could receive early postoperative intraperitoneal 5-fluorouracil using 600 mg/m2 for 4 days.
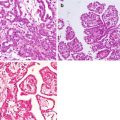
Histopathologic examination showed a well-differentiated mucinous adenocarcinoma (Fig. 3.5). The patient’s postoperative course was uneventful. The ileostomy was closed 1 year later. During this procedure, four small tumor nodules were visualized and removed without difficulty. In 2010, the patient had an abnormal CT 4 years after his definitive procedure. A repeat complete cytoreductive surgery (CCRS) was possible along with additional HIPEC. In 2014, a solitary recurrence in the epigastric region was shown on CT. Radiation therapy was used to control progression. However, in 2015 the mass again began to expand and interfere with gastric function. A left upper quadrant exenterative procedure with total gastrectomy was able to achieve complete resection. Currently, at 10-year post-first procedure, the patient is free of disease.
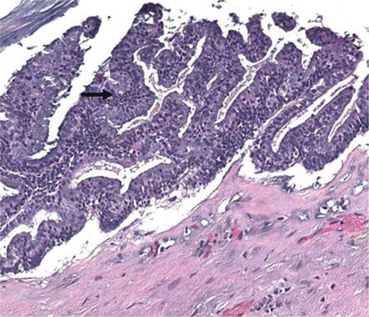

Fig. 3.5
Photomicrograph from a patient with urachal cancer of the wall of the primary tumor mass showed a well-differentiated mucinous malignancy (hematoxylin and eosin ×400) (From Sugarbaker [59])
3.2.2 Discussion
The medical literature was searched using PubMed for publications that reported a urachal adenocarcinoma concomitant with PM. The results of this survey are presented in Table 3.1 [7, 12, 19–28]. Mendeloff and McSwain recognized a direct relationship of mucinous PM and the urachal primary cancer [7]. Loggie et al. was the first to report a definitive treatment plan for the local-regional (LR) component of this disease [20]. In that patient, systemic metastases became evident at 20 months after diagnosis; the large-volume intraperitoneal component of the disease never recurred despite systemic progression of disease. Perioperative chemotherapy was used in the report by de Bree as well as in four of our five patients [12, 24]. The patient reported by de Bree et al. was alive and well at 9 years after treatment (personal communication).
Table 3.1
Literature review of urachal adenocarcinoma presenting with pseudomyxoma peritonei
Author | Year | N | Age/sex | Pathology | Cytoreduction | Cystectomy | HIPEC | Survival | CEA/CA 19-9 preoperative | Current status |
|---|---|---|---|---|---|---|---|---|---|---|
(months) | ||||||||||
Mendeloff [7] | 1971 | 1 | 49/M | Mucinous adenocarcinoma | Debulking | No | No | 28 | NA/NA | DOD |
Sasano [19] | 1997 | 1 | 45/M | Cystadenoma | None | No | No | NA | 5.7/135 | NA |
Loggie [20] | 1997 | 1 | 35/M | Signet ring | Extensive | Yes | Mitomycin C 40 mg | 31 | NA/NA | DOD |
De Bree [12] | 2000 | 1 | 34/M | Low-grade mucinous | Extensive | No | Mitomycin C | 108 | NA/NA | NED |
Yanagisawa [21] | 2003 | 1 | 50/F | Mucinous cystadenocarcinoma | Oophorectomy | No | No | NA | NA/NA | NED |
Stenhouse [22] | 2003 | 1 | 55/M | Low-grade mucinous | NA | No | No | NA | 28/NA | NA |
Takeuchi [23] | 2004 | 1 | 82/M | Mucinous adenocarcinoma | Minimal | No | No | NA | 7.9/38 | NA |
Sugarbaker [24] | 4 | 32/F | Mucinous adenocarcinoma | Extensive | No | EPIC | 132 | 4/182 | DOD | |
47/M | Mucinous adenocarcinoma | Extensive | No | Mitomycin C | 20 | 14/594 | NED | |||
51/M | Low-grade mucinosis | Extensive | No | No | 68 | NA/NA | DOD | |||
38/F | Adenocarcinoma | Extensive | No | Mitomycin C Doxorubicin | 13 | 171/10 | DOD | |||
Shen [25] | 2009 | 5 | NA | NA | NA | NA | NA | NA | NA | NA |
Glehen [26] | 2010 | 4 | NA | NA | NA | NA | NA | NA | NA | NA |
Barrios [27] | 2015 | 4 | NA | NA | NA | NA | NA | NA | NA | NA |
Sugarbaker [28] | 2015 | 1 | 59/M | Adenomucinosis | Extensive | No | Cisplatin Doxorubicin Ifosfamide | 10 | 15.6/94 | NED |
3.2.3 Diagnosis of Urachal Pseudomyxoma Peritonei
In the patients reported in the medical literature and in our own five patients, the urachal mucinous neoplasm presented the clinical picture of pseudomyxoma peritonei. The diagnosis of urachal mucinous adenocarcinoma is rarely made prior to the initial exploratory surgery; often, the necessary plans to definitively treat the disease are not formed. However, in the patient presented above, prior experience with this disease led to a diagnosis preoperatively, and definitive treatment with CRS and HIPEC occurred as a single event. The symptom of mucus noted upon urination should be recognized as an unusual complaint distinctively associated with this rare disease. Computed tomography showing a cystic mass anatomically related to the position of the urachus can also suggest the diagnosis. In some patients, this primary tumor mass may be noted prior to the development and progression of the extensive mucinous ascites described as pseudomyxoma peritonei. In our patient and others shown in Table 3.1, a greatly elevated CA 19-9 tumor marker was helpful in making a diagnosis of mucinous urachal PM and has been utilized in follow-up.
In patients who present with mucosuria and a cystic lower midline abdominal mass on CT or magnetic resonance imaging (MRI), a primary urachal adenocarcinoma should be suspected. During the prolonged course of this disease prior to diagnosis, the urachal mucinous neoplastic cells gain access to the peritoneal cavity. In this environment, they continue to disseminate as neoplastic cells in mucinous ascites moving with peritoneal fluid throughout the peritoneal cavity. This characteristic pattern of tumor dissemination associated with pseudomyxoma peritonei is known as “redistribution phenomenon” [2, 29]. In some patients, the mucus from the primary tumor mass is also forced down a patent urachus into the bladder, causing mucosuria. A definitive treatment approach to this disease using CRS and HIPEC similar to that used for patients with pseudomyxoma peritonei from an appendiceal mucinous neoplasm may be of greatest benefit to these patients.
3.2.4 Treatment of Urachal Pseudomyxoma Peritonei
Complete excision of a primary urachal adenocarcinoma with clear margins is the treatment of choice if primary cancer resection alone can achieve negative margins. The mucinous nature of this neoplasm and the possibility for stray cancer cells developing at a later time as pseudomyxoma peritonei must be considered. An en bloc resection of dome of the bladder and tumor is the preferred surgical strategy. The decision to proceed with a cystectomy versus simple resection of the superior aspect of the bladder with negative margins will depend on the anatomic extent of the disease and its biological aggressiveness. In the proper clinical setting, cystectomy is not mandatory [30]. In patients with pseudomyxoma peritonei arising in a primary urachal adenocarcinoma, the disease is associated with a cystic primary cancer that produces copious mucus. In this type of primary cancer, deep invasion into the bladder is less likely to occur. In our patients and in the others reported in the literature, cystectomy was not required. Although the primary urachal cancer is usually manageable by surgical resection, the peritoneal spread presents a special problem in management requiring CRS and HIPEC.
3.2.5 Follow-Up of Urachal Pseudomyxoma Peritonei
Most of these patients have an elevated CEA and CA 19-9 tumor marker at the time of diagnosis of the pseudomyxoma peritonei (Table 3.1). In our patients, the tumor markers increased with disease recurrence and declined to normal with CCRS. These tumor markers should be used in a serial manner in follow-up. These mucinous tumors are well imaged by CT, especially if the bowel is filled by oral contrast. We recommend for follow-up tumor markers CEA and CA 19-9 every 3 months and chest, abdominal, and pelvic CT every 6 months for 5 years post-cytoreduction. Similar recommendations have been made for appendiceal malignancies [31].
3.2.6 Pathology of Urachal Pseudomyxoma Peritonei
Urachal remnants are usually lined by transitional-type epithelium; however, focal glandular metaplasia may give rise to mucinous adenocarcinoma similar to that seen with colon cancer. These tumors are similar to appendiceal neoplasms in that there is a large spectrum of biological aggressiveness between patients. In some patients, the epithelial cells are described as bland, well differentiated, and noninvasive and are thought to be of borderline malignancy [22]. In other reports, an aggressive signet ring histomorphology is reported [19]. We have suggested that the term “mucinous urachal neoplasms” be used to describe this clinical entity to include this broad range of histologic types of noninvasive- as well as invasive-appearing tumors.
3.3 Pseudomyxoma Peritonei from Small Bowel Adenocarcinoma
3.3.1 Case Report
In January 1995, the patient developed intestinal obstruction. He was originally explored through an appendectomy incision and found to have mucinous small bowel adenocarcinoma. Through a midline incision, he underwent a right colon resection with resection of the primary colon in the terminal ileum. He was treated with six cycles of intraperitoneal 5-fluorouracil with systemic mitomycin C. He also had radiation therapy for progressive tumor within the appendectomy incision.
In January of 1996, the patient developed partial small bowel obstruction and was taken back to the operating room for excision of tumor from the abdominal wall with resection of the right rectus abdominis muscle and a redo right colon resection.
The patient did well for 2 years but then had recurrent small bowel obstruction, and in January 1998, he had a third resection. The patient was found to have extensive radiation fibrosis. An additional 3 ft of small bowel were removed along with resection of tumor in and along the right ureter. All specimens showed infiltrating metastatic mucinous adenocarcinoma. At the time of the third operation, the tumor had foci of poor differentiation with transmural involvement of the small bowel.
In February 1999, recurrent symptomatic tumor on the anterior abdominal wall was removed. In September 1999, hematuria developed and a cystoscopy showed mucinous adenocarcinoma infiltrating the wall of the bladder. Systemic chemotherapy and then best palliative care were initiated. The patient died in September 2000, 5 years and 9 months after his diagnosis.
3.3.2 Discussion
This patient demonstrates the gradual transition over time and with multiple interventions of tumor histology from low grade to higher grade. This change is associated with a less favorable outcome with repeated surgical interventions. The frequency of this change in the biology of a malignancy is not known but has been documented for pseudomyxoma peritonei of appendiceal origin [32].
Small bowel adenocarcinoma is a rare malignancy causing less than 5% of gastrointestinal cancer [33]. Only a fraction of small bowel adenocarcinoma patients will manifest pseudomyxoma peritonei. However, it does occur and can be successfully treated by CRS and HIPEC because of the relative sparing of the remainder of the small bowel by mucinous neoplasms [8]. The expected survival of small bowel adenocarcinoma with pseudomyxoma peritonei is not known. But a large multi-institutional experience with PM from small bowel adenocarcinoma is reported in the Monograph of the French Surgical Association [33]. They report on 45 patients who had a median survival of 32 months. The 1-, 3- and 5-year survival was 81%, 47%, and 33%, respectively. The clinical factors influencing survival were the experience of the institution treating the patient (p = 0.048) and the completeness of cytoreduction (p ≤ 0.001). The number of patients with mucinous peritoneal metastases in this experience was not reported.
Evaluation of this data regarding the efficacy of CRS and HIPEC is difficult because there is little or no information regarding the survival of patients with PM from small bowel cancer treated by surgery alone. Fishman et al. have reported the largest series treated with chemotherapy demonstrating a 30% response rate. The use of palliative chemotherapy in this setting of metastatic and locally advanced unresectable disease achieved a median survival of 11 months [34]. Currently, combinations of systemic chemotherapy combined with CRS and perioperative chemotherapy can be recommended in patients having mucinous peritoneal metastases from small bowel adenocarcinoma with a moderate to low PCI and the possibility of a complete cytoreduction.
3.4 Mesenteric Cyst Resulting in Pseudomyxoma Peritonei
3.4.1 Case Report
A 38-year-old woman was presented in June 2008 with diffuse mucinous ascites with intra-abdominal and pelvic neoplasm. Her initial symptoms of fatigue, urinary frequency, fever, weight gain, and moderate abdominal pain began after her pregnancy in 2003. The symptoms became more severe in December 2007, with increased abdominal fullness and pain. She sought medical advice and a diagnosis of pelvic inflammation was made. The antibiotic treatment she underwent was not helpful. A CT scan was performed which showed mucoid ascites in the abdomen and pelvis. An exploratory laparotomy was performed in January 2008. Mucoid ascitic fluid was drained during the surgical intervention. Copious mucoid tumor was found on the peritoneal surfaces, especially involving the greater and lesser omentum. A total abdominal hysterectomy, bilateral salpingo-oophorectomy, mesenteric cystectomy, omentectomy, and appendectomy were performed. A large mesenteric cyst was resected and is shown in Fig. 3.6a, b.
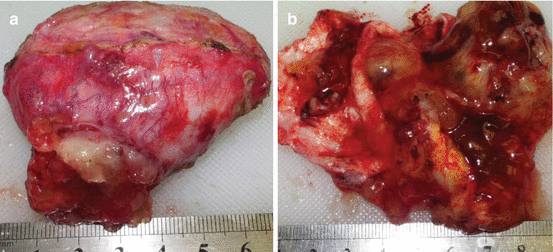

Fig. 3.6
Photograph of the mesenteric cyst removed at initial surgical intervention. (a) The cyst was perforated with mucus extruding from its surface. (b) The cyst was bisected to show its multilocular character and mucinous contents (From Sugarbaker [59], with permission)
Because of uncertainty regarding the etiology of the primary tumor, the pathology specimens were sent to other medical centers for review. An ovarian teratoma present in the resected specimen was not considered to be the primary site because it did not contain epithelium or mucinous material. However, the ruptured mesenteric cyst contained abundant borderline neoplastic mucinous epithelium. The diagnosis was pseudomyxoma peritonei originating from malignant transformation of a mesenteric cyst.
At our institution, the CT scan revealed residual mucinous tumor nodules at multiple sites within the abdomen and pelvis. Persistent tumor was layered out beneath the right hemidiaphragm, class 0 changes were present within the small bowel mesentery, and a mass had developed at the apex of the vagina.
The patient underwent a complete CRS followed by HIPEC plus 5-fluorouracil. Mitomycin C and doxorubicin were given by the intraperitoneal route and 5-fluorouracil and leucovorin by intravenous administration [35]. The pathology returned as disseminated peritoneal adenomucinosis (DPAM) (Fig. 3.7). The decision was made not to recommend any further treatment. The patient is asymptomatic and on a 6-monthly CT scan regimen for the next 5 years. She remains well with no evidence of disease at 6 years.
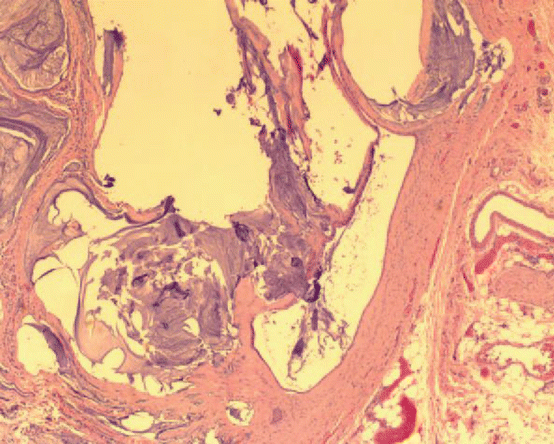

Fig. 3.7




Photomicrograph of tumor in a patient with a mesenteric cyst taken from the pelvis showed adenomucinosis. The peritoneal lesions showed simple mucinous epithelial strips with abundant extracellular mucin. Bland epithelium had no cytological atypia or mitosis (hematoxylin and eosin ×500) (From Sugarbaker [59], with permission)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree