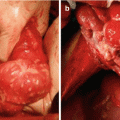
Fig. 5.1
Low- (a-5 and b-20×) and high-power (c-40×) histologic sections of DSRCT from an omental biopsy. In figure (c), nests of small round blue cells (filled arrow) interdigitate between bands of fibrous stroma (line arrow)
5.2 Diagnosis and Staging
The age of presentation is typically 5–30 years, and 85–90% of the patients are male [5].
Large masses, in addition to visceral and parietal seeding of the peritoneum, are a typical presentation in DSRCT. Usually vague abdominal pain brings this to the attention of the patient and prompts imaging examinations. The dissemination of DSRCT throughout the abdominal cavity is characteristic. The reason a large tumor burden exists at diagnosis is few symptoms are present until the peritoneal surfaces are infiltrated with tumor and overwhelm the peritoneum, therefore impairing resorption of peritoneal fluid and causing ascites. Abdominal distension and discomfort are the usual presenting symptoms. Patients can also have pain and constipation. Because of the sarcomatosis seen, these patients are considered Stage 4 at diagnosis. It is rare for a patient to present with a single mass or one or two masses. This only occurs when the mass is found incidentally at the time of another operation or diagnostic radiologic exam for another entity.
Because of the frequent diffuse nature of the presentation of this disease, a new staging system is being considered, and now being used on a trial basis, by Hayes-Jordan and colleagues at MD Anderson Cancer Center. In this proposed staging system, Stage 1 patients would have limited disease, localized to one or two sites in the abdomen or one site elsewhere. Stage 2 patients would have any amount of extensive peritoneal disease; Stage 3, with liver metastasis and peritoneal disease; and Stage 4 with peritoneal and liver disease and disease also outside of the abdominal cavity, including lymph nodes. This has not been validated and is under investigation.
5.3 Imaging Characteristics
On initial imaging, typically, CT (computed tomography) scans are done. MRI and ultrasound can also be helpful. On CT scan or MRI, usually multiple peritoneal implants can be seen, making the diagnosis of DSRCT highly suspicious. The most common site of initial organ metastasis is usually the liver. The lungs, pleura, and mediastinum are the next most common locations for metastasis. Lymph node enlargement in the groin and neck can also be seen. Therefore, PET (positron-emission tomography) scan imaging may be a helpful adjunct to evaluate distant metastasis at the time of staging [6].
The extent of disease seen on initial imaging includes many lesions in every portion of the peritoneal cavity. The most common areas are the omentum, right diaphragm, and pelvis (Fig. 5.2). The splenic hilum and various small bowel and colon mesenteric implants are also common. Retroperitoneal disease is very uncommon. In most cases, the disease seen on CT or MRI imaging underestimates the extent of the diseases. One to 2 mm metastasis and “sheets” of tumor in confluence are common intraoperative findings (Fig. 5.3). Metastatic disease outside of the abdominal cavity can be found in the mediastinum, pleura, supradiaphragmatic lymph nodes, lung, and bone.
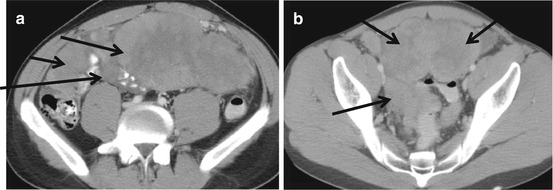
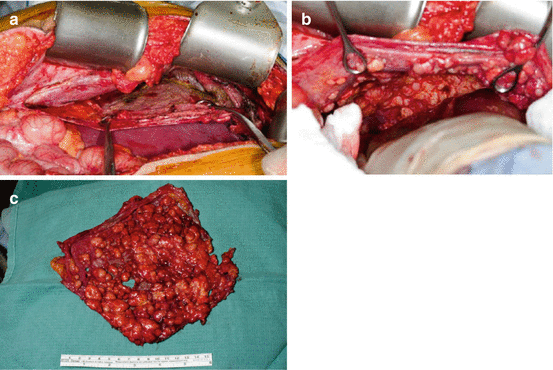

Fig. 5.2
Figure (a) shows a large omental mass in a newly diagnosed patient with DSRCT. Figure (b) shows a pelvic, paravesical mass, large and lobulated. Pelvic tumors are very typical of DSRCT sarcomatosis

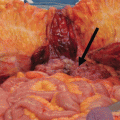
Fig. 5.3
A “sheet” of sarcomatosis from DSRCT in the right diaphragm peritoneum. Figure (a, b) show the intraoperative dissection of the right diaphragm peritoneum. The final result (c) is one “sheet” of tumor without any diaphragm muscle removed
5.4 Chemotherapy
Since its description in 1989 by Gerald and Rosai at Memorial Sloan Kettering Cancer Center, multimodality chemotherapy has been used for DSRCT. Ewing’s type chemotherapy, aggressive surgery, tumor debulking, total abdominal radiation therapy, and high-dose chemotherapy followed by autologous stem cell rescue have all been used in the treatment of DSRCT, with little improvement in survival. Durable remissions remain rare [7]. Control of DSRCT with chemotherapy is most effective in children, with Ewing’s type chemotherapy. Ewing’s type chemotherapy is the standard because efficacy with this regimen has been demonstrated by Kushner et al. [7]. This chemotherapy is based on alkylating agents cyclophosphamide or ifosfamide along with vincristine and doxorubicin alternating with ifosfamide and etoposide. This regimen was shown to have a favorable outcome in a multidisciplinary approach in 12 DSRCT patients [7]. This chemotherapy regimen was used in combination with aggressive surgical complete excision and postoperative whole abdominal radiation, providing improved survival. With a median follow-up of 22 months, the median disease-free survival was 19 months. The regimen can be quite toxic, and frequent admissions for fever and myelosuppression can be expected. An alternative more tolerable outpatient regimen has been utilized [8]. This includes neoadjuvant vincristine, ifosfamide, dexrazoxane/doxorubicin, and etoposide. This is followed be aggressive surgical excision and removal of all gross disease, including 1–2 mm peritoneal implants. This was followed by adjuvant radiotherapy (30 Gy whole abdomen) and irinotecan and Temodar for a total of 12 cycles. This regimen yielded a disease-free interval of approximately 2 years. The irinotecan and Temodar therapy provided an excellent quality of life with regular school attendance and participation in plan activities. This regimen may be used after surgery and radiotherapy [8].
5.5 Surgical Therapy
As mentioned, abdominal sarcomatosis is a common finding with tumor implants ranging from 1 mm to 40 cm or more. The extent of disease seen on initial imaging includes many lesions in every portion of the peritoneal cavity. Typically, omental disease is found in most patients in addition to peritoneal studding on the diaphragm, spleen, Morison’s pouch, abdominal wall peritoneum, small bowel mesentery, and almost certainly in the pelvis. Peritonectomies are required in these locations for effective complete gross resection and cytoreduction. In most cases, the disease seen on CT or MRI imaging underestimates the extent of the diseases. One to 2 mm metastasis and “sheets” of tumor in confluence are common intraoperative findings.
Because this is usually a very chemo-responsive tumor, the feasibility of surgical resection should not be assessed until a plateau of response from chemotherapy has been reached. This is usually achieved after 4–6 months of neoadjuvant chemotherapy. The partial response to neoadjuvant chemotherapy in DSRCT is an important component to complete surgical resection. In a report of the impact of complete surgical resection of DSRCT, LaQuaglia and colleagues found a 3-year overall survival of 58% with complete resection and 0% when resection was not done, and the patients were treated with chemotherapy and radiotherapy alone [5].
In this setting, even after surgical resection of gross, visible disease, and cytoreduction, microscopic residual can be expected. Hence, a regional approach to local control such as hyperthermic intraperitoneal chemotherapy (HIPEC) could be an effective strategy for DSRCT. HIPEC is a potential adjunct to complete surgical resection of DSRCT. Figure 5.4. shows a schemata of a typical HIPEC setup, including the infusion of heated chemotherapy (41.5 °C) which occurs over a 90-min period in the operating room after complete cytoreduction (Fig. 5.4).
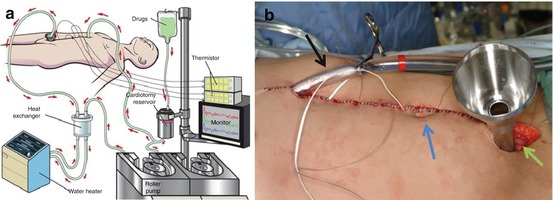

Fig. 5.4
(a) A representation of the HIPEC technique with a simple pump that pumps the heated chemotherapy into the abdominal cavity and recirculates, in a closed technique, over 90 min in the operating room, using cisplatin for chemotherapy in the case of DSRCT. (b) The closed abdomen of a patient after cytoreduction, ready to begin HIPEC. Temperature probes can be seen exiting from the midline skin closure that will be attached to a computer to provide a constant monitoring of the intra-abdominal temperature. Black arrow denotes inflow catheter, green arrow is outflow port, and blue arrow is the umbilicus of a supine patient
Complete surgical resection, including cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for carcinomatosis, is standard therapy for appendical carcinoma and pseudomyxoma peritonei, among others [9–16]. Complete cytoreduction and HIPEC have been found to improve survival in many studies of carcinomatosis [14, 18–20]. Intraperitoneal therapy is currently the recommended approach in carcinomatosis of ovarian and mesothelioma origin [2, 17–23]. In the context of a prospective randomized trial, gastric cancer patients with carcinomatosis underwent cytoreduction accompanied by normothermic or hyperthermic mitomycin C. The overall 5-year survival of surgery alone, normothermic, or hyperthermic perfusion was 42%, 43%, and 61%, respectively [2]. In ovarian carcinoma, significantly superior survival has been found in the intraperitoneal chemotherapy group compared to intravenous cisplatin and paclitaxel in a national prospective randomized trial [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree