Blood Collection
In the United States, 14.2 million red blood cells and whole blood donations and approximately 2.4 million apheresis and whole blood–derived platelets were collected in 2013. Although whole blood donations are the primary method for the manufacturing of red blood cells (RBCs), plasma, and cryoprecipitate components, platelets are now predominantly collected by apheresis technology. Individual component manufacturing allows for the optimal storage conditions for each component, as well as individualizing transfusion therapy by allowing for replacement of only those components that the patient clinically requires rather than transfusing whole blood.
The blood collection process begins with cleaning the phlebotomy site thoroughly with 2% chlorhexidine gluconate and 70% isopropyl alcohol for 30 seconds, followed by 30 seconds of drying time to prevent introduction of skin bacteria into the collection system. Fig. 19.1 shows the numbered steps in the collection process. After a large-bore needle ( 1 ) is placed into an antecubital vein, the initial flow of blood is directed to a diversion pouch ( 2 ) to decrease the risk of bacterial contamination by diverting the skin plug cored during insertion of the needle, which may contain deep-seated bacteria that may evade the cleaning process. After the diversion pouch is filled (this blood is used for infectious disease marker [IDM] testing), it is sealed off and blood flows into the main collection bag ( 3 ), which contains citrate phosphate dextrose (e.g., CPD or citrate phosphate double dextrose [CP2D]).
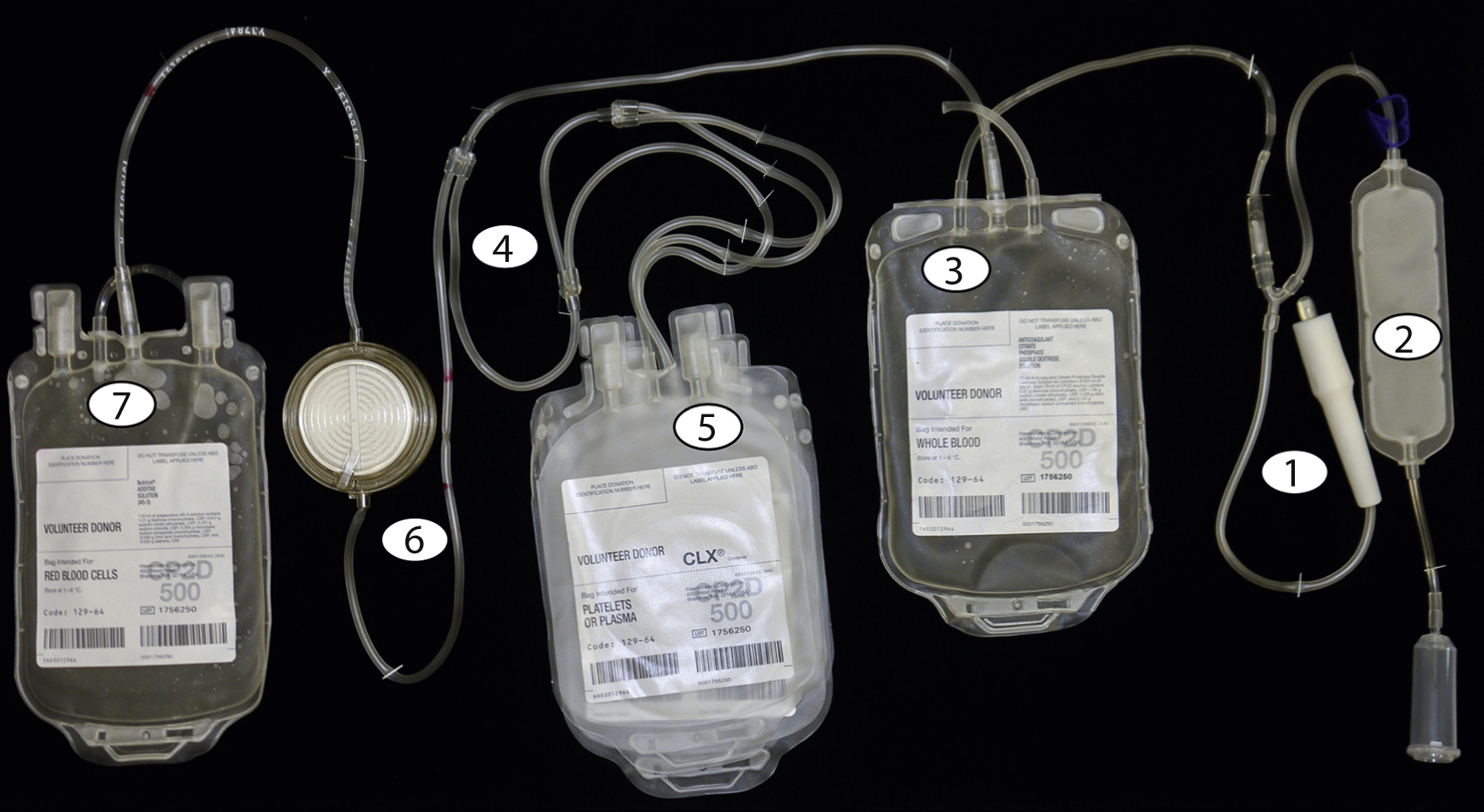
The sodium citrate provides anticoagulation and the phosphate and dextrose maintain red cell metabolism and adenosine triphosphate (ATP) production. Other additive solutions containing adenine, mannitol, and electrolytes can be added later to extend the shelf life of the red cells.
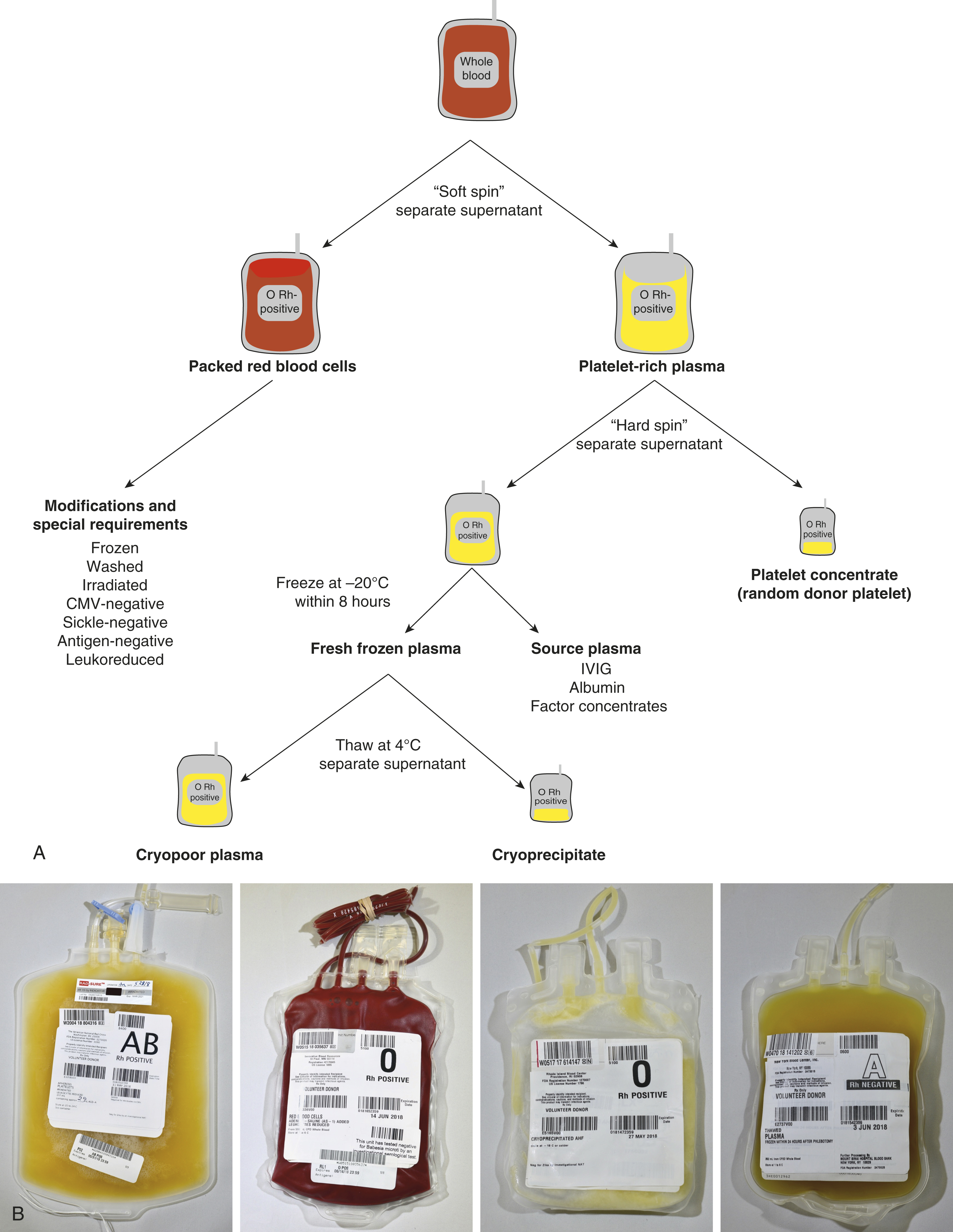
The main collection bag is then placed into the centrifuge and given a “soft spin” (1500 g for 5 minutes) to pellet the red cells and leave platelet-rich plasma (PRP) as the supernatant. The PRP is then expressed off and directed ( 4 ) into one of the attached component bags ( 5 ). The RBCs that remain in the collection bag are then expressed off and directed ( 4 ) through a leukoreduction (LR) filter ( 6 ) into the final RBC collection bag containing anticoagulant. Further processing in a closed system ( Fig. 19.2 ) is performed to prepare additional components without introducing contaminants.
Blood Components
After a whole blood collection, the main collection bag containing whole blood is centrifuged at 1500 g for 5 minutes (soft spin) to produce RBCs that pellet to the bottom of the bag and PRP as the supernatant. The PRP is then expressed into one of the attached component bags.
The packed red cells that remain subsequently flow by gravity through an LR filter into the final RBC collection bag. Anticoagulant-preservative solutions CPD or CP2D allow for a 21-day shelf life, whereas citrate-phosphate-dextrose-adenine (CPDA) contains adenine in addition to citrate, phosphate, and dextrose, thereby extending the shelf life to 35 days. If additive solutions (AS-1, AS-3, AS-5) containing additional adenine, dextrose, and sodium chloride are added to the packed red cells, the shelf life can be extended from 21 or 35 days to 42 days. AS-1 and AS-5 contain mannitol but AS-3 does not.
The component bag containing the PRP is centrifuged at 5000 g for 10 minutes (“hard spin”) with the pellet composed of platelets and the supernatant composed of platelet-poor plasma (PPP). The plasma is expressed off into another component bag, and if the plasma is frozen within 6 to 8 hours of collection, the product can be labeled as fresh frozen plasma (FFP). If the plasma is frozen within 8 to 24 hours, it can be labeled as plasma frozen within 24 hours (FP24). These products may be thawed and transfused within 24 hours. If FFP or FP24 is thawed and kept for greater than 24 hours, the component is then relabeled as thawed plasma (TP), which must be transfused within 5 days of the initial thaw. The platelet pellet is then resuspended in a small amount of plasma to make a whole blood–derived platelet concentrate, also known as a random donor platelet (RDP) unit.
If the FFP is removed from the freezer and thawed at 4 ± 2°C, a cryoprecipitate forms and can be pelleted by centrifugation. The supernatant is cryopoor plasma (cryosupernatant) and the pellet can be resuspended to make a unit of cryoprecipitate. Contrary to common belief, cryoprecipitate is not concentrated plasma, but rather most of the product is composed of fibrinogen, factor VIII, von Willebrand factor, factor XIII, and fibronectin. Alternatively, the plasma can be processed as “source plasma” to make coagulation factor concentrates, albumin, intravenous immunoglobulin, and other plasma-derived products.
Apheresis
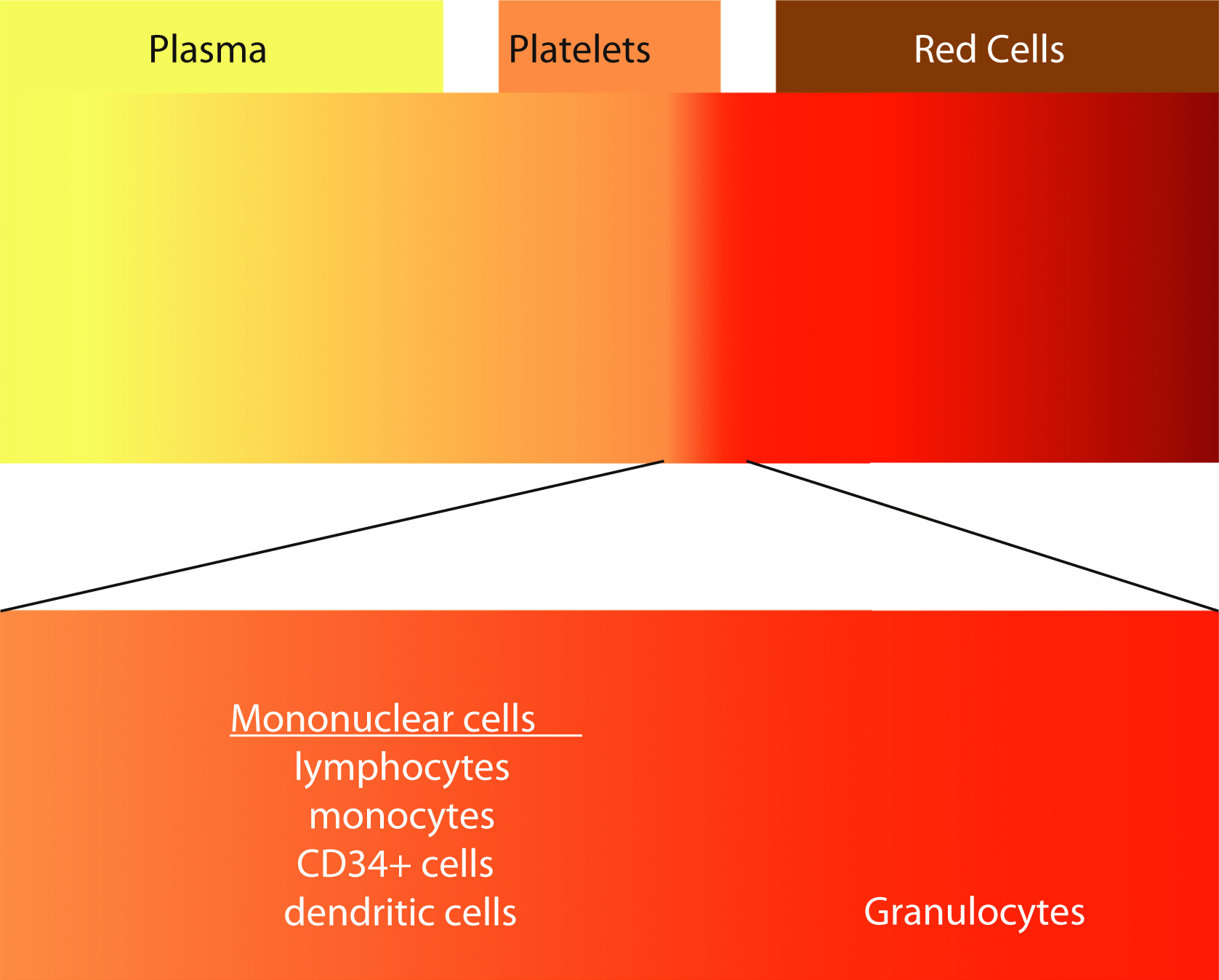
Although most blood components are prepared from processing of whole blood, the use of apheresis technology to collect blood components has been increasing. In the United States, platelets collected by apheresis has increased from 58% of total platelet products in 1999 to 93.9% in 2015. , Apheresis is derived from the Greek word aphairesis , which means “a taking away.” The procedure separates the components of whole blood by centrifugation. The whole blood separates into layers, with RBCs having the highest specific gravity, plasma having the lowest, and platelets, granulocytes, and mononuclear cells in between ( Fig. 19.3 ). Each of these layers can be selectively collected using a single-use, disposable, and sterile kit using an automated instrument. The advantage of apheresis collections over whole blood collection is the ability to collect multiple components from a single procedure. Two units of RBCs or 2 to 4 units of plasma or 2 to 3 units of platelets or combinations of RBCs, plasma, and platelets from a single donor can maximize low prevalence blood types. For example, red cells can be preferentially collected from type O–negative donors, whereas plasma and platelets from type AB donors would be selected. Additional advantages include more frequent allowable donations for the platelet and plasma donors. Platelet donors can donate twice a week with at least 2 days between donations with a maximum of 24 times per year.
Pediatric Units
Transfusion of blood components to pediatric patients should be based on the consideration of individual patient laboratory values and symptoms and not only transfusion triggers. Unfortunately, there is a lack of high-quality evidence to guide transfusion. Unlike adults for whom the blood is ordered in units, pediatric patients are frequently much smaller and doses are weight based. For example, an apheresis platelet dose for a 15-kg child would be 10 mL/kg (150 mL). Blood units are usually aliquoted into smaller bags ( Fig. 19.4 ) or a syringe before issue to provide the dose requested. For example, a unit of RBCs can be aliquoted into three smaller aliquots by sterilely docking the main unit with the aliquot kit using a sterile machine that melts and fuses the tubing from the RBC unit to the aliquot bags. This maintains a closed system and does not change the expiration date of the product. This method optimizes the volume transfused and decreases donor exposures by providing aliquots from the same unit as needed.
Leukoreduction
LR of packed RBCs and platelets has been shown to reduce febrile nonhemolytic transfusion reactions (FNHTR), human leukocyte antigen (HLA) alloimmunization, and platelet refractoriness in patients receiving multiple transfusions and prevent transfusion-transmitted cytomegalovirus (CMV). Although many in the blood bank community believe that LR reduces transfusion-related immune modulation (TRIM) and improves patient outcomes (e.g., decreased overall mortality, decreased length of stay, decreased risk of surgical site wound infections), this view is still controversial. As the number of hematology/oncology patients continues to grow, so will the challenge of providing HLA-matched and cross-matched platelets to patients with alloimmune platelet transfusion refractoriness. As such, many institutions have elected to provide universal LR products to their patients.
Prestorage LR ( Fig. 19.5 ) can be performed by the blood processing facility shortly after collection by allowing the RBC or pooled platelet component to flow by gravity through an LR filter. LR can also be performed at the patient bedside but may not be as effective because the leukocytes can release cytokines during storage, which can still lead to FNHTRs. Current third-generation LR filters can remove 99.99% (4-log reduction) of leukocytes with less than 10% red cell loss. Apheresis platelets are leukoreduced via the apheresis collection process. Current U.S. Food and Drug Administration (FDA) guidelines and AABB (formerly American Association of Blood Banks) Standards for Blood Banks and Transfusion Services require that RBCs, apheresis platelets, and pooled platelets contain 5 × 10 6 or fewer leukocytes in the final product. Whole blood–derived platelets are required to contain fewer than 8.3 × 105 leukocytes. Quality control standards require that 95% or more of sample units pass the standard.
Irradiation
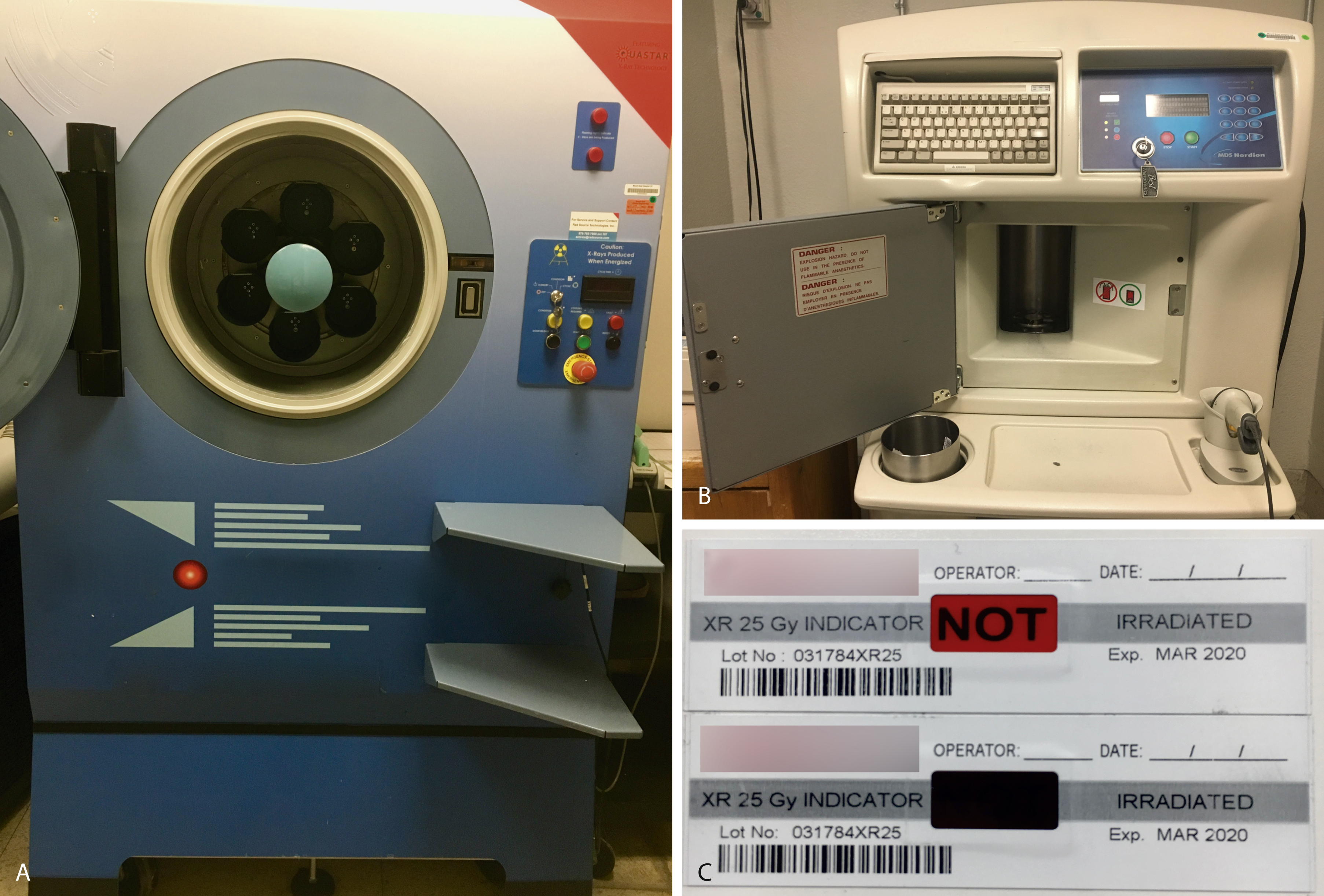
Transfusion-associated graft-versus-host disease (TA-GVHD) occurs approximately 4 to 30 days after transfusion of a blood component with immunocompetent lymphocytes (e.g., packed RBCs, granulocytes, platelets) to an at-risk patient who is unable to reject donor lymphocytes (e.g., hematopoietic transplant, intrauterine transfusion, congenital immunodeficiency). In addition to immune deficiencies, communities with low HLA diversity (e.g., Japan), where unidirectional HLA disparity will more often occur, are at increased risk. In at-risk recipients, the donor lymphocytes (graft) engraft, proliferate in the patient (host), and attack host tissues. The median time of onset of symptoms is 10 days; patients initially present with fever, skin lesions (maculopapular rash, blisters, ulcers), gastrointestinal symptoms (e.g., diarrhea, vomiting, gastrointestinal bleeding), cholestasis, or pancytopenia. Unlike GVHD after a hematopoietic transplant, which has a 10% to 25% mortality rate, the rate of mortality of TA-GVHD is 90% to 100%. The incidence is unknown owing to underrecognition, underreporting, and overlap with other reactions. TA-GVHD is a clinical diagnosis, but detection of donor lymphocytes can help confirm the diagnosis. Leukoreduction is not sufficient to prevent TA-GVHD. γ-Irradiation (25 Gy) ( Fig. 19.6 ) offers complete protection by inactivating lymphocytes by cross-linking DNA. γ-Irradiation can be produced using nuclear material such as x-rays ( panel A in Fig. 19.6 ) or cesium-137 ( panel B ). Indicator strips (panel C) are used to confirm that the dose of γ-irradiation was appropriate. However, irradiation damages red cells (e.g., increased potassium leakage) and shortens the shelf life to 28 days or the original expiration date, whichever is shorter. Photochemical pathogen inactivation with amotosalen plus ultraviolet light exposure is an alternate strategy for prevention of TA-GVHD.
Blood Storage
Red blood cells are stored in anticoagulant-preservative solutions containing sodium citrate, citric acid, dextrose, phosphate (CPD, CP2D), CPDA-1 (CPD with adenine), and additive solution formulas (AS-1, AS-3, AS-5, AS-7). Most collection facilities in the United States collect 450 mL ± 10% (405–495 mL) from a whole blood donor. Red cell components prepared from whole blood are approximately 300 to 350 mL in volume with 200 to 250 mL of red cells in the final product. Red cells are stored at 1°C to 6°C and are transported at 1°C to 10°C. The hematocrit, volume, and expiration date vary according to the anticoagulant-preservative storage medium. LR does not change the expiration date of packed red cells. However, other red cell modifications may require modification of the expiration date. Irradiation can cause damage to the red cells, such as the release of potassium and free hemoglobin into the supernatant. For this reason, the expiration of an irradiated red cell is shortened to 28 days or the preirradiation expiration date, whichever is shorter. When rare blood cells are frozen in 40% glycerol within 6 days of collection, they can be stored at −65°C for 10 years. When thawed for transfusion, red cells are deglycerolized using a multistep washing process. If the washing system is open, then the expiration is set at 24 hours and if it is a closed system, the expiration is 14 days, or as FDA-approved. Washed red cells are washed with saline solution and are often used for patients with a history of repeated allergic reactions to plasma proteins. The washing process also removes residual leukocytes and platelets. When washed in an open system, packed red cells expire in 24 hours ( Table 19.1 ).
| Components | Hematocrit | Expiration |
|---|---|---|
| CPD, CP2D | 65%–85% | 21 days |
| CPDA-1 | <80% | 35 days |
| Additive solutions (e.g., AS-1, AS-3, AS-5) | 55%–65% | 42 days |
| RBCs, irradiated | — | Original expiration date or 28 days from date of irradiation, whichever is sooner |
| Deglycerolized frozen red cells Washed red cells | 50%–80% | 24 hours (open system) 14 days (closed system) |
| Frozen red cells, 40% glycerol | NA | 10 years |
Whole blood–derived or apheresis non-RBC components include platelets, plasma, granulocytes, and cryoprecipitate ( Table 19.2 ). One of the advantages of blood component therapy over whole blood transfusion is the ability to store the components under optimal storage conditions. Platelets are best stored at room temperature because chilled platelets are rapidly cleared from the circulation. However, room temperature storage allows proliferation of bacteria that may be present, potentially causing transfusion-transmitted bacteremia or sepsis. Platelets are also optimally stored with gentle agitation to allow optimal gas-exchange with the suspension. Plasma contains several labile coagulation factors such as factor V and factor VIII that are best preserved by freezing plasma within 6 to 8 hours of collection (FFP) and storing frozen for 1 year at or below −18°C or 7 years if stored at or below −65°C. Similarly, the coagulation factors in cryoprecipitate are best preserved frozen after being manufactured from FFP.
| Component | Volume | Storage | Transport | Expiration |
|---|---|---|---|---|
| Apheresis platelets, ± leukoreduced, ± irradiated | 250–300 mL | 20°C–24°C with continuous agitation | 20°C–24°C | 5 days |
| Random donor (whole blood–derived) platelets | 50 mL | 20°C–24°C with continuous agitation | 20°C–24°C | 5 days |
| Pooled platelets | ~300 mL | 20°C–24°C with continuous agitation | 20°C–24°C | 4 hours after pooling |
| Fresh frozen plasma | 250 mL | ≤ −8°C or ≤ −65°C | Frozen | ≤ −18°C: 12 months from collection ≤ −65°C: 7 years from collection |
| Fresh frozen plasma, thawed | 250 mL | 1°C–6°C | 1°C–10°C | 24 hours |
| Thawed plasma | 250 mL | 1°C–6°C | 1°C–10°C | 5 days from date product was thawed |
| Cryoprecipitate or pooled cryoprecipitate | 15 mL | ≤ −18°C | Frozen | 12 months from collection |
| Thawed cryoprecipitate | 15 mL | 20°C–24°C | 20°C–24°C | 6 hours (closed) or 4 hours (open) from date/time product was thawed |
| Granulocytes | 300–400 mL | 20°C–24°C | 20°C–24°C | 24 hours |
Infectious Disease Marker Testing
One of the main goals of the blood banking community and regulatory agencies is to ensure a safe blood supply. The FDA, which regulates blood as a pharmaceutical, requires that infectious disease testing be performed on each donation. Donors are first screened with a thorough medical history questionnaire and subsequent interview and an abbreviated physical examination consisting of blood pressure, pulse, and temperature. The infectious risk has been reduced by screening for infection, (even those that are not currently tested for), high-risk sexual activity and travel to areas at high risk for bovine spongiform encephalopathy. If the history and physical screening is passed, then blood is collected as part of the donation process for IDM testing. Table 19.3 provides a list of tests required for the infectious disease testing. Some blood donor collection facilities extend the testing menu for agents that are not required by the FDA. IDM testing for Babesia microsti is required in high-risk states (i.e., New York, Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, Rhode Island, Vermont, Wisconsin, and Washington, DC). If a first-time donor has positive results for one of the IDMs, confirmatory testing is performed and, if confirmed, then the donor is deferred from donating in the future. If the donor is a repeat donor, then the donor will be deferred, confirmatory testing is performed, and a lookback procedure will notify recipients of blood from that donor. Although CMV testing is one of the IDMs, a positive test result does not defer a donor. Instead, the CMV-seronegative designation provides an extra safety measure for vulnerable populations (e.g., fetuses, neonates, and bone marrow [BM] transplant recipients when the donor and recipient are both CMV negative). The blood banking community has significantly changed the residual risk of transfusion transmitted infection as the result of the advances in IDM testing methods. The introduction of nucleic acid testing has reduced the window period for human immunodeficiency virus (HIV) detection to 11 days. Table 19.4 shows the residual risk of transfusion based on the window period of the test (the time from the infection to the ability to detect the infection) and the sensitivity/specificity of the testing system.
| Infectious Agent | Tests |
|---|---|
| Hepatitis B virus | HBsAg, anti-HBc, nucleic acid test |
| Hepatitis C virus (HCV) | Anti-HCV, nucleic acid test |
| Human immunodeficiency virus (HIV) 1/2 | Anti-HIV 1/2, nucleic acid test HIV-1 |
| Human T-lymphotropic virus (HTLV) I/II | Anti-HTLV I/II |
| Treponema pallidum (syphilis) | Anti- T. pallidum |
| Trypanosoma cruzi (Chagas disease) | Anti- T. cruzi |
| West Nile virus | Nucleic acid test |
| Cytomegalovirus a | Anti-CMV |
| Zika virus | Nucleic acid test |
| Babesia a | Nucleic acid test |
| Infectious Agent | Residual Risk |
|---|---|
| Hepatitis B virus | 1:500,000 to 1:1,000,000 |
| Hepatitis C virus | 1:1,148,628 |
| Human immunodeficiency virus 1/2 | 1:1,466,671 |
Blood Group Antigens
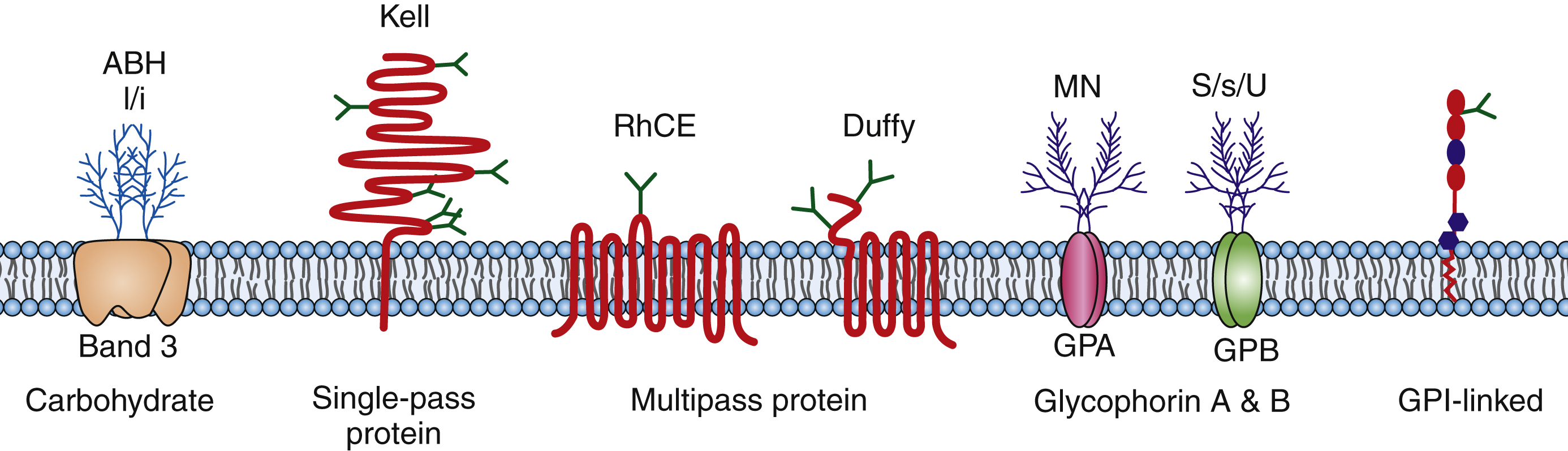
Blood group antigens are markers on the surface of red blood cells that are composed of protein and carbohydrates ( Fig. 19.7 ). Blood group antigens and their corresponding antibodies are important because they are the major immunologic barrier to safe transfusion and are also involved in the pathophysiology of human disease (e.g., hemolytic disease of the fetus/newborn [HDFN]). The ABO blood group system is the most well-known blood group system and was first described by Landsteiner in 1900. The A and B antigens are carbohydrate antigens that are codominantly expressed on the RBC surface and determine the blood type (type A, B, AB, O). In addition to ABO, there are 29 blood group systems as described by the Committee on Terminology for Red Cell Surface Antigens of the International Society of Blood Transfusion. Some of the more common and clinically significant blood groups include the Rh, Kell, Duffy, Kidd, and MNSs blood group systems. Red cell antigens are composed of carbohydrates and proteins that are part of the cell membrane. The ABO and I/i antigens are carbohydrates attached to the lipid bilayer or proteins (glycolipids, glycoproteins). The Rh, Kell, Duffy, and Kidd blood groups are protein antigens. Kell is a single-pass membrane protein; RhD and RhCE antigens are multipass (six passes) membrane proteins with C- and an N-terminals that are intracellular; Duffy is also a multipass (seven passes) protein with a C-terminus that is intracellular and an N-terminus that is extracellular. Finally, proteins can also be linked to the cell membrane through glycophosphatidylinositol (GPI) links such as the Dombrock blood group system. Many of these antigens are part of structures that have known functions. Rh protein complex may act as an ammonium transporter; Kidd is a urea transporter; Colton is carried on aquaporin-1, which is a channel for water; and Indian is carried on CD44 and is a single-pass membrane protein that appears to be the major human hyaluronan receptor. Other blood groups are on structures that function as enzymes and complement regulatory proteins.
Blood Group Antigens and Their Clinical Significance
Blood group antigens are frequently identified after causing either a hemolytic transfusion reaction (HTR), delayed or acute, or HDFN. These reactions may vary from mild to severe and may even be fatal. Some reactions are clinically insignificant, only causing positive results on pretransfusion testing. The severity of the reaction seen can vary among individuals, and reaction severity is predicted by evaluation of published case reports, prior history of reactions in that patient, strength of the reaction in testing or presence of hemolysis on testing, temperature at which reactions occur, and whether the immunoglobulin (Ig) G or IgM antibodies are identified. Table 19.5 summarizes Ig classes and the predicted likelihood and severity of HTRs and HDFN for the most commonly identified antigenic specificities.
| Blood Group Antigens | Immunoglobulin Class | Clinical Significance | |
|---|---|---|---|
| HTR | HDFN | ||
| Rh | |||
| D | IgG | Red | Can cause reactions |
| C | IgG | Red | Cause of rare or generally mild reactions |
| c | IgG | Red | Can cause reactions |
| E | IgG | Red | Cause of rare or generally mild reactions |
| e | IgG | Red | Cause of rare or generally mild reactions |
| Kell | |||
| K | IgG | Red | Can cause reactions |
| k | IgG | Red | Can cause reactions |
| Kidd | |||
| Jk | IgG, IgG, and IgM | Red | Can cause reactions |
| Jk | IgG, IgG, and IgM | Red | Cause of rare or generally mild reactions |
| Duffy | |||
| Fy | IgG | Red | Can cause reactions |
| Fy | IgG | Red | Cause of rare or generally mild reactions |
| MNS | |||
| M | IgG (usually cold reacting) and IgM | Green | Does not cause reactions |
| N | IgG (usually cold reacting) and IgM | Green | Does not cause reactions |
| S | IgG and IgM | Yellow | Cause of rare or generally mild reactions |
| s | IgG and IgM | Yellow | Reactions are rare but may be severe |
| Lewis | |||
| Le | IgM | Yellow | Does not cause reactions |
| Le | IgM | Green | Does not cause reactions |
Immunohematology
Autoimmune antibodies are antibodies that bind to antigens that are expressed on an individual’s own red cells and usually also bind to most donor red cells. Alloantibodies are antibodies that are formed when an individual is exposed to an antigen that they do not express either by red cell transfusion, pregnancy, or organ transplant. They are generally referred to as “irregular” antibodies. The major exception to alloantibody formation is anti-A and anti-B antibodies that are formed in type A, B, and O individuals despite the lack of exposure to A and B red cell antigens. The current belief is that the ABO blood group system is an exception because individuals are exposed to bacterial cell walls in the environment that closely resemble the structure of the A or B antigen, which stimulates the anti-A and/or anti-B formation. Red cell alloantibodies can be a major immunologic barrier to transfusion, and red cells lacking the corresponding antigen should be provided to prevent an acute or delayed HTR.
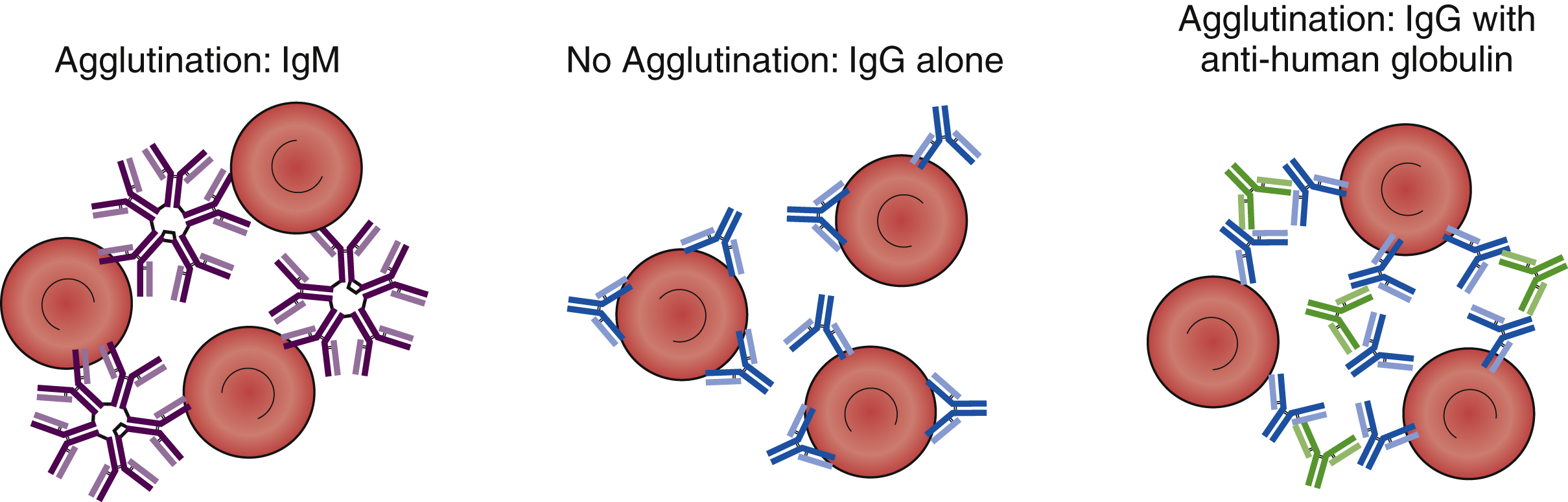
The clinical significance and serologic reactivity of antibodies depend on the immunoglobulin type (e.g., IgM, IgG, IgA, IgE) and the subtype (e.g., IgG1, IgG2, IgG3, IgG4). The majority of the antibodies involved in immunohematology pretransfusion testing and transfusion reactions are IgG and IgM antibodies. These antibodies have characteristics, although there are exceptions. IgG antibodies are smaller than IgM antibodies because they are a monomeric immunoglobulin with two Fab sites, whereas IgMs are pentamers with 10 Fab binding sites. Because IgMs are larger with a greater number of binding sites, IgM can directly agglutinate red cells. For example, anti-A and anti-B are IgM antibodies that can directly agglutinate A and/or B red cells. IgG antibodies, however, are not large enough and do not have enough binding sites to directly bridge red cells. To achieve agglutination, an anti-human globulin is needed to bridge the IgG Fc domains. With the exception of anti-A and anti-B antibodies, IgM antibodies are not clinically significant when they are only cold reactive, and most IgM antibodies are only cold reactive. IgG antibodies are generally clinically significant and cause extravascular hemolysis. That is because IgG is too small to allow complements to form the membrane attack complex (MAC), whereas IgM can assemble the MAC ( Table 19.6 ).
IgM antibodies are large, multivalent antibodies that can bridge individual red cells, and they can product direct agglutination when added to red cell antigens that they are directed against ( Fig. 19.8 ). In contrast, IgG antibodies are bivalent and too small to bridge red cells and produce direct agglutination. To produce agglutination, anti-human globulin (anti-IgG [AHG]) must be added to bridge the individual IgG antibodies coating the individual red cells. When red cells are coated in vivo by IgG antibodies (e.g., anti-D in HDFN), agglutination is not observed in vitro. However, if AHG is added, then agglutination will be observed. The addition of AHG to red cells coated in vivo by IgG antibodies is called the direct antiglobulin test (DAT) or the direct Coombs test. It is used to provide in vitro evidence of HDFN, warm autoantibodies, and HTR. In vitro testing for antibodies may require the addition of an IgG antibody against the target (e.g., addition of anti-K to phenotype a red cell). However, agglutination will not occur because the IgG cannot bridge the red cells. A second step, the addition of AHG, is required to visualize agglutination. This is called the indirect antiglobulin test (IAT) or the indirect Coombs test and is used in RBC antibody identification panels and RBC antigen phenotyping in pretransfusion testing.