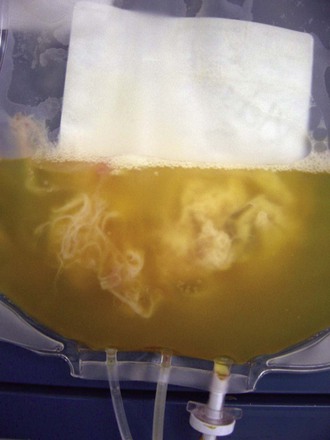
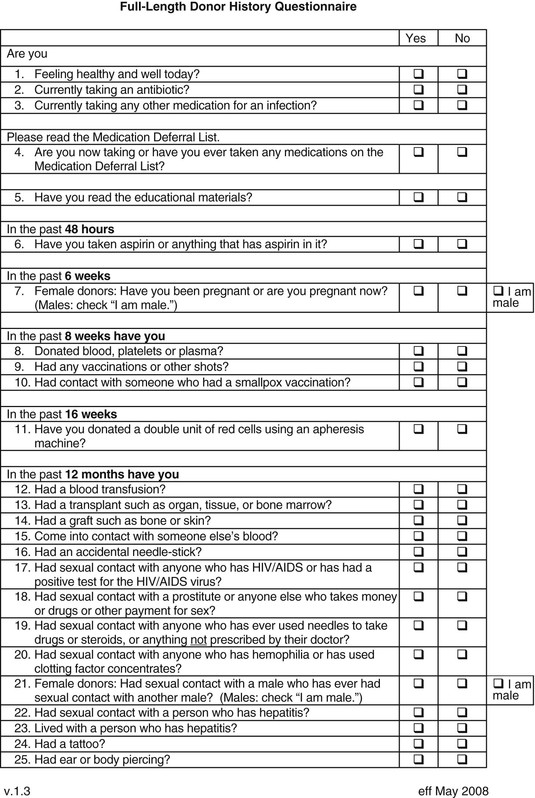
Matthew J. Kuehnert, Sridhar V. Basavaraju * Beeson reported the first cases of transfusion-associated infection in 1943, describing seven patients who developed hepatitis from 1 to 4 months after receiving a red blood cell (RBC) or plasma transfusion.1 The advent of the acquired immunodeficiency syndrome (AIDS) epidemic in the 1980s followed by recognition of transfusion transmission of hepatitis C virus (HCV) and, most recently, documentation of the association of the West Nile virus (WNV) epidemic with transfusion-transmitted infection have continued to draw attention to the safety of the blood supply, particularly the potential risk from emerging infectious agents.2–4 Although infections with the human immunodeficiency virus (HIV), HCV, and now WNV have been the most publicized, a wide spectrum of other organisms, including viruses, bacteria, parasites, and prions, have been transmitted by transfusion (Table 306-1).5 Of these, bacteria transmitted by transfusion, often associated with platelets, occurs most commonly and may infrequently result in sepsis and death.6 Recognition of the threat of Trypanosoma cruzi transmission via transfusion and the availability of suitable testing have led to screening for this pathogen, which is the cause of Chagas disease. In addition to WNV, mosquito-borne pathogens, such as dengue and chikungunya viruses, have shown potential for transfusion transmission, although the burden of disease is unknown. Tick-borne agents also are recognized to pose an increasing risk to transfusion safety, including transmission of babesiosis and, most recently, anaplasmosis and ehrlichiosis. Transmission of variant Creutzfeldt-Jakob disease (vCJD) via transfusion has occurred in the United Kingdom. TABLE 306-1 List of Notable Infections Transmitted by Blood Transfusion Viruses Cytomegalovirus Colorado tick fever virus Dengue virus Epstein-Barr virus Hepatitis A virus Hepatitis B virus Hepatitis C virus Hepatitis E virus Human herpesvirus 8 Human immunodeficiency virus 1 and 2 Human T-lymphotropic virus 1 and 2 Parvovirus B19 Tickborne encephalitis virus West Nile virus Bacteria Anaplasma phagocytophilum Brucella spp. Coxiella burnetii Ehrlichia Gram-positive organisms* Gram-negative organisms† Rickettsia rickettsii Treponema pallidum‡ Parasites Babesia spp. Leishmania spp. Trypanosoma cruzi Plasmodium spp. Prions Variant Creutzfeldt-Jakob disease * Higher risk in platelets (see Table 306-3); additionally, Streptococcus gallolyticus (bovis) is notable for being associated with colon cancer in the donor. † Noted in red blood cell units associated with cryophilic organisms, including Pseudomonas fluorescens, Serratia liquefaciens, and Yersinia enterocolitica. ‡ Not thought a current risk; last reported transfusion transmission of syphilis in the United States was in 1966. Modified from Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080-2099; and Stramer SL, Hollinger FB, Katz LM, et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49:1S-29S. An ongoing dilemma of the blood-banking community is a mandate to ensure a maximally safe blood supply while giving consideration to the cost of such measures. Pathogen reduction of blood components, using methods that inactivate the nucleic material present in most viruses and bacteria, has been proposed to obviate the ever-increasing list of needed screening tests, but no such pathogen reduction technology has been approved for use in the United States. The current questions for screening donors that have been adopted by most blood banks are presented in Figure 306-1, and the laboratory screening measures currently in place are listed in Table 306-2. As of 2014, pathogens or diseases screened solely through donor interview include babesiosis, malaria, and vCJD. Real or perceived threats to the blood supply occur regularly, as emerging or reemerging infectious diseases raise concern over potential transmission. A recent review summarizes potential risks to the blood supply by pathogen, the majority of which remain unscreened by laboratory testing.7 TABLE 306-2 Laboratory Screening Performed for Pathogens or Diseases by U.S. Blood Collection Centers Required or Recommended by U.S. Food and Drug Administration Hepatitis B surface antigen (HBsAg) Hepatitis B core antibody (anti-HBcAb) Hepatitis C virus antibody (anti-HCV) HIV-1 and HIV-2 antibody (anti-HIV-1 and anti-HIV-2) HIV p24 antigen HTLV-1 and HTLV-2 antibody (anti-HTLV-1 and anti-HTLV-2) Serologic test for syphilis NAAT for HIV-1, HBV, and HCV NAAT for West Nile virus Serologic test for Trypanosoma cruzi (one-time donor testing) Additionally Required by Accrediting Organizations* Platelet screening for bacterial contamination† Performed Voluntarily Serologic test for cytomegalovirus (on request) * Including AABB (formerly known as the American Association of Blood Banks) and the College of American Pathologists. † Generally, liquid culture media for apheresis units and pH/glucose indicators or bacterial antigen point-of-use tests for whole-blood–derived pooled units. HBV, hepatitis B virus; HIV, human immunodeficiency virus; HTLV, human T-cell lymphotropic virus; NAAT, nucleic acid amplification testing. According to estimates from the World Health Organization (WHO), more than 92 million units of blood are collected in the world annually. Less than half of donated blood is collected in developing and transitional countries, which are home to about 80% of the world’s population. Of the 164 countries providing data to the WHO, 39 were not able to screen all of their donated blood for one or more of the four infections (HIV infection, hepatitis B, hepatitis C, and syphilis) that are most widely recognized to be transmitted through blood and are recommended by the WHO to be screened at donation. A total of 106 countries have national guidelines on the appropriate clinical use of blood, whereas 57 countries have a national hemovigilance system to monitor adverse events associated with transfusion.8,9 Surveys to determine blood product use in the United States, led by the National Heart and Lung Institute (now called the National Heart, Lung and Blood Institute), began in 1971.10 In the United States, surveys have reported the frequency of blood collection and utilization since the late 1980s, most recently through the Nationwide Blood Collection and Utilization Survey (NBCUS), which has been conducted biannually since 1998.10–13 In the 2013 NBCUS report, reflecting data collected in 2011, there were approximately 13.7 million whole-blood and 3.3 million apheresis collections, with more than 20 million blood components transfused.13 Each recipient received an average of 2.75 units of blood. In 2011, 13.8 million whole-blood and RBC units, 3.9 million units of fresh-frozen plasma, 2.0 million units of apheresis platelets, 0.2 million units of whole-blood–derived platelets (i.e., measured in apheresis equivalent units), and 1.0 million units of cryoprecipitate were given.13 The number of RBC transfusions has declined significantly since 2008, perhaps reflecting the growing adoption of more aggressive hospital blood management practices. The number of transfused platelets has remained approximately the same, but transfusion of apheresis platelets has increased while the amount of whole-blood–derived or pooled platelets has declined dramatically in the same interval. In addition to contributions from the voluntary donor pool, plasma units also are collected annually from paid donors and are used to prepare immune globulin, albumin, and various other plasma-derived products.13 By 1987, the cost of collecting, processing, and transfusing patients exceeded $3 billion; since then, costs have increased steadily with the addition of new screening tests and the implementation of leukoreduction.10 In 2011, the average price paid by a hospital was $225 for a unit of leukocyte-reduced RBCs, $58 for fresh-frozen plasma, and $535 for apheresis platelets, so current costs are likely to exceed $5 billion annually.13 It has been estimated that the annual likelihood of an individual’s receiving a transfusion is about 0.89% and increases dramatically with age; in 2011, the transfusion rate in the U.S. population was 44 units per 1000 people.13,14 Because of concern about contracting an infectious disease, there has been historic interest in autologous and donor-directed blood donation. However, donor-directed units, usually given by family members for a specific patient, have been shown to have higher rates of various infectious agents. Recently, there has been movement away from donor-directed donation and toward building a dedicated, voluntary repeat donor population.12,13 Viral infections are much less common among repeat donors compared with first-time whole-blood donors, and they may be even less common among donors associated with apheresis collection.15,16 A majority of blood components transfused in the United States are leukocyte reduced, a modification that is performed to filter out the majority of white blood cells. Leukoreduction is performed to reduce nonhemolytic transfusion reactions, but it may also reduce the risk for infectious disease transmission through removal of infected white blood cells, particularly for cell-associated agents.17 In 2011, only about 0.7% of the donated allogeneic blood supply was discarded on testing, usually because of detection of a potentially transmissible infection.13 This reflects a continued steady decline in units discarded due to testing, perhaps indicating improved accuracy of screening tests and retention of repeat donors. The incidence of viral markers in donated units was increased among volunteers who donated after September 11, 2001, primarily owing to a large cohort of first-time and infrequent repeat donors.18 Transfusion-associated transmission risk has persisted for HIV, hepatitis B virus (HBV), HCV, and human T-cell lymphotropic virus type 1 (HTLV-1) for two distinct reasons: (1) the incomplete sensitivity of the available screening tests and (2) the “window” period, which is defined as the period between acute infection (and potential infectivity) and the time when serologic tests can reliably detect infection. The development and implementation of routine nucleic acid amplification testing (NAAT) has transformed blood bank screening for viral pathogens.19–21,22 NAAT can detect viral RNA after the first 10 to 14 days of infection, narrowing the window period by 7 to 10 days for HIV and by 50 to 60 days for HCV compared with serology. Additional NAAT has been developed for blood donor screening, including tests for WNV, HBV, and parvovirus B19.23 With introduction of NAAT, the risk for acquiring HIV or HCV per unit of blood transfused has plummeted from approximately 1 per 500,000 for HIV and 1 per 100,000 for HCV before NAAT to a current rate of 1 per 2 million for both viruses. Detected cases are so unusual that the incidence has to be estimated statistically.24 As remarkable as this advance is, a small window period remains, meaning that the risk for receiving one of these viruses in a unit of blood is not zero. Four cases of HIV transmission and many more cases of HCV transmission through transfusion have been recognized and reported to public health authorities since NAAT was implemented; the modeling data estimate more cases than the number reported, so some cases likely go unrecognized.25 The advent of NAAT also has had a substantial impact on the choice of serologic tests. In addition to sensitivity, blood bankers seek optimal specificity: false-positive or indeterminate tests exclude otherwise appropriate donors from subsequent donations and create anxiety due to incorrect diagnosis of infection. The introduction of an extremely sensitive test such as NAAT allows blood banks to focus on the specificity of other screening tests, thereby limiting the number of persons potentially excluded from the donor pool because of misleading test results. Although sensitivity varies little between the second- and third-generation antibody tests, the third-generation assay has superior specificity, decreasing the proportion of persons with indeterminate antibody tests for HCV.26 HBV NAAT is now being adopted for blood donor screening. More than 8000 persons in the United States have developed AIDS from receipt of blood or tissue.27 In addition, at least 50% of all hemophiliacs in the United States and Europe became infected with HIV from 1978 to 1985, most from receipt of infected plasma factors, with the highest incidence in 1982, when there were 22 infections per 100 person-years.28 The first HIV screening test was introduced in 1985, and since 1987 few new infections among hemophiliacs have been reported.28 As noted, the introduction of NAAT screening has substantially lowered the risk for HIV transmission, with the last reported case occurring in 2008. Redundant methods of testing also have been useful in confirming the status of infection for blood donor follow-up. NAAT has been useful in clarifying the HIV serostatus of persons with indeterminate results of Western blot tests.29 These reactions occur in about 1 of every 5000 donations and usually represent false-positive tests.30 In June 1992, the U.S. Food and Drug Administration (FDA) mandated screening for HIV-2.31 Since then, few HIV-2–positive donors have been identified, and no cases of transmission have occurred in the United States, although transfusion-related HIV-2 cases have occurred elsewhere.32,33 HTLV-1 and HTLV-2, unlike HIV-1 and HIV-2, are cell associated and therefore predominantly transmissible only with blood component transfusions.34 Screening for both types is done with a single test. The rate of transmission of HTLV-1 decreased almost 10-fold (from 1 per 8,500 to 1 per 69,000) after introduction of the screening test.35 Transplantation of solid organs from an HTLV-1–infected donor resulted in rapid progression to subacute myelopathy in three recipients; this phenomenon has not been described in transfusion recipients.36 A short-lived perceived threat to the blood supply was raised when xenotropic mouse leukemia virus–related virus (XMRV) was linked to prostate cancer and then chronic fatigue syndrome. Furthermore, evidence of viral infection, either by serology or NAAT, was found in controls, including in over 5% of healthy blood donors. XMRV later was proved to be a laboratory contaminant and is not thought to be associated with pathogenic human infection.37 HBV remains the virus with the highest residual risk of transfusion transmission despite screening; HBV is transmitted in approximately 1 in every 200,000 to 300,000 transfusions.38,39 Risk persists both because the screening test is incompletely sensitive and because of the prolonged window period of about 2 months. In countries that screen for hepatitis B core antibody (HBcAb), most transmissions derive from the window period, whereas in those that do not check HBcAb status, half derive from the window period and the others are due to the insensitivity of the HBsAg test as a screening test.8 NAAT for HBV recently has been implemented for blood screening using minipool testing. With current sensitivity and minipool testing, NAAT shortens the window period by about 7 days, leaving a residual risk for HBV of about 1 per 250,000 to 350,000 transfusions.39,40 Sensitive screening tests are particularly needed in HBV-endemic areas, such as Taiwan, where HBcAb testing is not routinely performed and transfusion-acquired HBV is a relatively frequent event.41 Testing for HBcAb, in addition to HBsAg, remains important; in some cases, it is more sensitive than NAAT, so implementation of new testing does not obviate the use of these other tests for donor HBV infection.42 Increasing use of hepatitis B vaccination may further reduce the risk for transmission. Screening of donors for HBsAg excludes most, but not all, carriers of delta virus.43 Identification of donors with antibody to the delta agent is not done routinely, so there is a residual risk for transmission of delta virus to HBsAg-positive recipients. Hepatitis C, previously known as “non-A, non-B hepatitis,” was associated with a lifetime risk of 7% to 10% in transfusion recipients in the late 1970s and early 1980s.44 The transmission rate of hepatitis C has decreased with improved screening tests to identify the virus, providing an excellent example of how implementation of each new generation of screening technology has improved blood safety. More than 90% of recipients of HCV-contaminated blood products develop HCV infection.45 An older survey using the second-generation test found that 3.6 per 1000 U.S. donors were positive for HCV,46 a prevalence lower than that in the U.S. population. HCV infection in blood donors usually is the result of intravenous drug use, although other nosocomial risks have been identified, including questionable injection practices. Before any testing, 0.45% of all transfusions transmitted HCV. With the introduction in 1986 of surrogate marker testing (alanine aminotransferase and HBcAb), the rate decreased to 0.19%, and with the introduction of the first-generation antibody test for HCV in 1990, the rate fell to 0.03%. The second-generation test lowered the rate even further, mostly by identifying chronic infections more accurately (i.e., increasing sensitivity) rather than by shortening the window period. Despite the continuing relatively high prevalence of HCV infection in the U.S. population, the introduction of NAAT has reduced the transmission risk to about 1 in 1.5 million transfusions.20,21,24 WNV has been transmitted by blood transfusion to at least 32 persons since this route of transmission was first recognized during the summer of 2002.4 In addition, it has been transmitted by organ transplantation.47 In response to this emerging threat, an NAAT test for WNV was quickly developed and implemented by 2003 and is now in routine use for blood donor screening; thousands of WNV-infected donations have been interdicted.22,23 Because of the logistic and financial burden of individual testing, WNV screening assays test pools of 6 or 16 donations (i.e., minipools), with follow-up screening of positive minipools by individual sample NAAT. However, “breakthrough” cases of WNV transfusion transmission still occur, because minipool testing is not as sensitive as individual sample NAAT. In response, blood banks are using strategies to “trigger” a switch from minipool to individual NAAT in areas with high epidemic activity during seasonal peaks.48 Twelve transfusion transmissions have occurred despite use of minipool NAAT.49 There have been five cases after blood collection agencies voluntarily put in place processes to trigger more sensitive individual donation testing based on local WNV activity. There have been no recognized cases of transfusion transmission when individual donation testing has been used, although one transmission involved a positive minipool with false-negative confirmatory individual test.50 WNV has also been transmitted through leukocyte transfusion.51 Clinicians should suspect WNV in recently transfused or transplanted individuals who develop unexplained encephalitis or flaccid paralysis. Immunosuppressed patients, including solid-organ and stem cell recipients, are at greatest risk for developing complications. Human herpesvirus 8 (HHV-8), the cause of Kaposi sarcoma, is believed to be primarily white cell associated, suggesting a low risk for transmission via leukoreduced blood transfusion, and few cases of transmission have been documented. The low rate of recognized transmission may reflect, in part, the difficulty in test performance and the high positive background rate, but studies have suggested a link.52,53 One epidemiologic study completed in Uganda estimated the rate of transmission to be 2.8% for nonleukoreduced RBC units, with excess risk associated with storage times shorter than 4 days.54 Further studies are needed to determine whether leukoreduction can completely mitigate this risk but it is more likely partially effective, as viral loads in free plasma have been found in infected individuals with Kaposi sarcoma.55 Transmission of HHV-8 resulting in Kaposi sarcoma has been described more frequently in recipients of solid-organ transplants.56 The transmission risk for cytomegalovirus (CMV) has been well summarized.57 CMV, similar to HHV-8, is highly cell associated and can be transmitted with white blood cells that persist in RBC or platelet transfusions. Transmission of CMV from fresh-frozen plasma or cryoprecipitate has not been reported. The insensitivity of the serologic test has resulted in a residual risk for transmission of 0% to 6%, even when CMV-seronegative donors are used.57 Because the demand for CMV-seronegative blood outstrips the supply, approaches such as leukocyte filtration of CMV-seropositive blood are often used, although the two approaches may not be equivalent in reducing CMV transmission risk.17 Screening for Epstein-Barr virus and other herpesviruses are not performed routinely, despite sporadic reports of transfusion-associated transmission. Parvovirus B19 has been transmitted in coagulation factor concentrates. The risk for transmission to cryoprecipitate recipients may persist despite treating with solvent and detergent and heating to 100° C after lyophilization.58 An epidemiologic study showed a significant correlation between receipt of treatment products, B19 seropositivity, and joint range-of-motion limitations.59 Transmission of hepatitis A virus (HAV) via transfusion has been described with RBCs, particularly in infants,60,61 and with factor VIII concentrate.62 Donors with early, asymptomatic infection may transmit HAV infection.63 Factor VIII–associated transmission has occurred despite appropriate use of organic solvent and detergent to inactivate the virus. The lack of a lipid envelope around HAV may have contributed to incomplete virus killing during preparation of the concentrate. Because of this small but persistent risk, vaccination for hepatitis A has been recommended for chronic recipients of products made from pooled plasma.64 Review of all cases of hepatitis from 1998 to 2002 in the United States among persons with bleeding disorders revealed no acquisition of HAV via factor concentrates.65 Hepatitis E virus (HEV) is endemic in many developing countries and is believed to have similar epidemiology to HAV with transmission via the fecal-oral route. HEV has been implicated in transfusion transmission worldwide but has not yet been reported in the United States.66,67 However, high seroprevalence rates nationally, especially in approximately 18% of donors in one study, have raised the concern about an underappreciated blood safety risk, and studies are underway for assessment.68 The search for the cause of non–A-E hepatitis has yielded many contenders but no indisputable etiology to date. Hepatitis G virus (HGV) has been found in 1% to 7% of donors and can be transmitted by transfusion.69 The risk for transmission is in the range of 5.3 per 10,000 units.70 However, HGV has been shown not to be a cause of non–A-E hepatitis, and the clinical implications of HGV infection remain undetermined.69 Two, somewhat related, single-stranded, unencapsulated DNA viruses, called transfusion-transmitted virus (TTV) and SEN virus (designated by the initials of the first patient investigated) have been considered as causes of non–A-E hepatitis.71,72 Both viruses are present worldwide and are often found in the blood of persons with post-transfusion non–A-E hepatitis. However, after initial interest, no causal association was established. Infusion of blood products contaminated by bacteria is a relatively frequent and potentially lethal risk of blood transfusion.5,73 As the risk for transfusion-transmitted viral infection has decreased dramatically, the frequency of transfusion-transmitted bacterial infection has remained unchanged. A possible exception to this persistent risk is transfusion transmission of Treponema pallidum, the etiologic agent of syphilis, which has not been reported to have occurred for decades, presumably because of poor spirochete survival under current storage conditions.74–76 Bacterial contamination may arise from donation, processing, storage, or transfusion and can result in transfusion-transmitted sepsis and death. Because platelets are stored at room temperature, they are at higher risk for bacterial growth than RBCs, which are stored at refrigerated temperatures. Bacterial contamination of blood components has been the most frequent cause of transfusion-related fatalities reported to the FDA after hemolytic reactions, accounting for more than 10% of transfusion-associated fatalities between 1985 and 1999. Although visual inspection can sometimes reveal a significantly contaminated unit, this is not always the case (Fig. 306-2).
Transfusion- and Transplantation-Transmitted Infections
Transfusion-Associated Infections

Scope of Blood Transfusion
Bloodborne Pathogens
Human Immunodeficiency Virus Type 1
Human Immunodeficiency Virus Type 2
Human T-Cell Lymphotropic Virus Types 1 and 2
Other Retroviruses
Hepatitis B and D Viruses
Hepatitis C Virus
West Nile Virus
Herpesviruses
Parvovirus B19
Hepatitis A and E Viruses
Non–A-E Hepatitis
Bacterial Pathogens
Transfusion- and Transplantation-Transmitted Infections
306