Toxicity
Sunitinib
Sorafenib
Pazopanib
Axitinib
All grade %
Grade 3/4 %
All grade %
Grade 3/4 %
All grade %
Grade 3/4 %
All grade %
Grade 3/4 %
General toxicities
Fatigue
31
5
33
3
14
3
39
11
Anorexia/asthenia
22
2
4
3
22
2
21
5
Infections
37
10
27
5
n/r
n/r
n/r
n/r
Gastrointestinal toxicities
Nausea
15
0
37
2
26
<1
32
3
Vomiting
12
0
19
2
21
2
24
3
Diarrhea
17
1
27
1
62
3
55
11
Mucositis/stomatitis
14
1
20
1
<10
<1
15
1
Dermatologic toxicities
Rash
25
<1
n/r
n/r
<10
<1
13
<1
Hand-foot syndrome
n/r
n/r
n/r
n/r
<10
<1
27
5
Cardiovascular/respiratory toxicities
Hypertension
30
12
17
4
13
<1
40
16
LVEF decrease
13
3
n/r
n/r
n/r
n/r
n/r
n/r
Dyspnea
10
2
14
4
n/r
n/r
n/r
n/r
Pneumonitis
n/r
n/r
n/r
n/r
n/r
n/r
n/r
–
Hematologic toxicities
Anemia
79
8
8
3
n/r
n/r
35
<1
Neutropenia
77
18
n/r
n/r
34
1
6
1
Thrombocytopenia
68
9
n/r
n/r
32
<1
15
<1
Laboratory/metabolic toxicities
Hyperglycemia
n/r
n/r
n/r
n/r
41
<1
n/r
n/r
Hypercholesterolemia
n/r
n/r
n/r
n/r
n/r
n/r
n/r
n/r
Hypertriglyceridemia
n/r
n/r
n/r
n/r
n/r
n/r
n/r
n/r
Hypophosphatemia
31
6
n/r
n/r
34
4
13
2
Hyperbilirubinemia
19
1
n/r
n/r
36
3
n/r
n/r
Hypercreatinemia
66
1
n/r
n/r
<10
<1
55
0
Increase in AST
52
2
n/r
n/r
53
7
n/r
n/r
Increase in ALT
46
3
n/r
n/r
53
12
n/r
n/r
Hypothyroidism
14
2
n/r
n/r
<10
<1
19
<1
Table 22.2
Selected treatment-related toxicities of mTOR inhibitors reported in phase III trials: everolimus and temsirolimus
Toxicity | Everolimus | Grade 3/4 % | Temsirolimus | Grade 3/4 % |
|---|---|---|---|---|
All grade % | All grade % | |||
General toxicities | ||||
Fatigue | 31 | 5 | 33 | 3 |
Anorexia | 22 | 2 | 4 | 3 |
Infections | 37 | 10 | 27 | 5 |
Gastrointestinal toxicities | ||||
Nausea | 15 | 0 | 37 | 2 |
Vomiting | 12 | 0 | 19 | 2 |
Diarrhea | 17 | 1 | 27 | 1 |
Mucositis/stomatitis | 14 | 1 | 20 | 1 |
Dermatologic toxicities | ||||
Rash | 25 | <1 | – | – |
Hand-foot syndrome | – | – | – | – |
Cardiovascular/respiratory toxicities | ||||
Hypertension | – | – | – | – |
LVEF decrease | – | – | – | – |
Dyspnea | 24 | 7 | 28 | 9 |
Pneumonitis | 14 | 4 | – | – |
Hematologic toxicities | ||||
Anemia | 92 | 12 | 45 | 20 |
Neutropenia | 14 | 0 | 7 | 3 |
Thrombocytopenia | 23 | 1 | 14 | 1 |
Laboratory/metabolic toxicities | ||||
Hyperglycemia | 57 | 15 | 26 | 11 |
Hypercholesterolemia | 77 | 4 | 24 | 1 |
Hypertriglyceridemia | 73 | <1 | 27 | 3 |
Hypophosphatemia | 37 | 6 | – | – |
Hyperbilirubinemia | 3 | <1 | – | – |
Hypercreatinemia | 46 | <1 | 14 | 3 |
Increase in AST | 21 | <1 | 8 | 1 |
Increase in ALT | 18 | <1 | – | – |
22.4 Fatigue and Asthenia
Fatigue and asthenia represent some of the most frequently encountered targeted agent-related side effects [2, 6, 7, 27–29]. Fatigue may be acute or chronic and is characterized by extreme tiredness and inability to function due to lack of energy. Asthenia includes weakness, lack of energy, and strength. Approximately 50–70 % of mRCC patients complain about fatigue, although only 5–10 % experience severe fatigue interfering substantially with the activities of daily living. Pazopanib appears to have a lower incidence of fatigue as compared to sunitinib and sorafenib although direct prospective comparisons are lacking [2, 30].
It is important to differentiate between drug-related fatigue, cancer-related fatigue, and fatigue related to other conditions (see below). It remains unclear what percentage of fatigue is cancer related and related to other conditions and what is treatment associated, since all types of fatigue are often coexistent in mRCC patients and difficult to differentiate in clinical practice.
To date, the mechanisms for cancer-related and sunitinib-induced fatigue are still poorly understood. A clearer understanding of the molecular mechanisms causing targeted agent-related fatigue would allow more targeted treatment, which might enable better maintenance of drug levels throughout treatment.
In studies, sunitinib-related fatigue was highly variable in both degree and duration. It appeared more common in men, particularly in young men, previously treated patients, and patients with repeated treatment interruptions. Typically, it occurred 2–3 weeks after treatment starts, increased in intensity during weeks 3 and 4, and tended to improve during the 2-week-off-treatment period [31]. There did not appear to be an increase in intensity of fatigue/asthenia with increasing number of treatment cycles but rather a decrease. Whether this phenomenon represents an adaptation and learning process by the patient or a true lower incidence remains unclear.
Alternative causes for fatigue should be ruled out before fatigue is attributed to treatment. This includes underlying dehydration, hypothyroidism, hypercalcemia, insomnia, anemia, pain, or depression. Fatigue improves in some patients who have received antidepressants or methylphenidate [32]. Heart failure and decreased left ventricular ejection fraction (LVEF) can also be associated with fatigue.
It is important to educate patients about fatigue, its symptoms, and potential tools to manage fatigue when it presents. Providing patients with written handouts about side effects, their prevention, and side effect management prior to initiating treatment is useful.
Very few evidence-based interventions to treat fatigue exist. Significant fatigue/asthenia interfering substantially with quality of life may be best managed by changes in dose and schedule as discussed above. General principles in the treatment of fatigue are shown in Fig. 22.1. The minimum recommendations for exercise include resistance training or aerobic exercise three times a week for 30 min. Recent randomized trials demonstrate better response in patients using resistance training [33]. The role of psychostimulants and nutritional supplements such as L-carnitine, melatonin, and American ginseng remains controversial with little existing evidence [34–36].
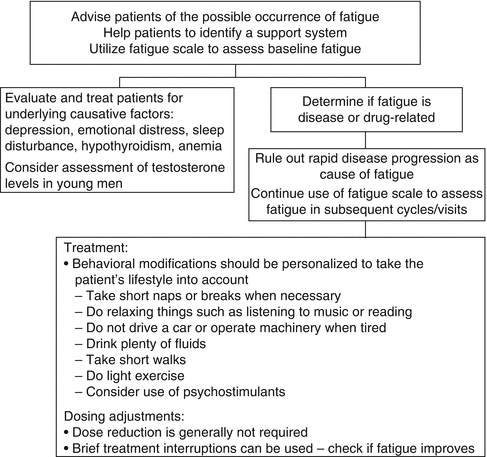

Fig. 22.1
Recommendations for fatigue management
22.4.1 Hypothyroidism
Hypothyroidism has been reported with all VEGFR TKIs. One or more thyroid function test abnormalities developed in up to 85 % of mRCC patients treated with sunitinib or axitinib [37–41]. There is a substantial discrepancy between incidence rates reported in early prospective trials (lower incidence) and some retrospective series or phase II studies (higher incidence), most likely due to infrequent testing for hypothyroidism in earlier studies, before hypothyroidism was recognized as a common side effect.
The presentation of thyroid dysfunction includes thyroid-stimulating hormone (TSH) elevation only with normal T4 levels (subclinical hypothyroidism), TSH elevation, and low T4 (overt hypothyroidism) that is more likely to be associated with clinical features of hypothyroidism, and even brief episodes of temporary thyrotoxicosis due to thyroiditis, often followed by hypothyroidism, have been described [39, 42, 43].
The exact primary mechanism by which hypothyroidism is caused remains unknown. Several hypotheses have been proposed including direct action of VEGFR TKIs on the VEGFR in the thyroid, induction of a destructive thyroiditis as suggested by the absence of visualized thyroid tissue preceded by TSH suppression, as well as endothelial dysfunction, regression of fenestrated capillaries, inhibition of iodine uptake, and reduced synthesis of thyroid hormone [37, 38, 40, 42, 44].
Hypothyroidism has been reported in patients receiving sunitinib as early as 1–2 weeks after initiation of therapy [42]. TSH tends to improve during the 2-week-off-treatment period. In the sunitinib studies, incidence tended to increase over time, while severity did not seem to increase with cycles. In retrospective series, up to 80 % of sunitinib-treated patients with abnormal thyroid function tests developed symptoms consistent with hypothyroidism such as fatigue, anorexia, edema, fluid retention, or cold intolerance. Thyroid hormone replacement clinically benefited only about 40–50 % of patients treated suggesting additional mechanisms for these side effects [37].
Interestingly, progression-free and overall survivals have been suggested to be improved in patients who experience hypothyroidism compared with euthyroid patients (10.3 months vs. 3.6) indicating that hypothyroidism may be a predictive factor for outcome [42, 45]. A positive correlation between hypothyroidism and improved clinical outcome has also been observed in breast cancer, brain cancer, and head and neck cancers. Importantly, there is no clinical data indicating that treatment of overt hypothyroidism worsens the outcome [45, 46].
Patients undergoing TKI therapy should undergo regular thyroid function monitoring (Fig. 22.2) [38]. All patients showing symptoms of overt hypothyroidism should be treated with thyroid hormone replacement therapy. Levothyroxine doses should allow normalization of TSH concentrations and resolution of symptoms. Those with asymptomatic subclinical hypothyroidism may be followed without levothyroxine therapy and treated when and if overt hypothyroidism develops. However, subclinical hypothyroidism was recently linked to a significant increase in risk for coronary heart disease events and mortality, indicating that hypothyroidism should be carefully observed and managed [47]. TKI-induced hypothyroidism is generally well manageable, and treatment interruptions or even treatment discontinuation or dose modifications for thyroid dysfunction are usually not necessary. It is important to continue monitoring and thyroxin supplementation after patients come off Rx with TKIs since hypothyroidism does not always resolve off TKI therapy.
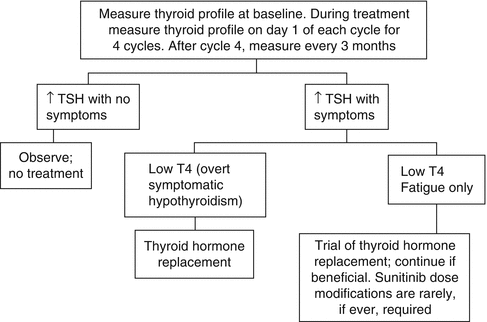

Fig. 22.2
Recommendations for thyroid dysfunction management
22.5 Skin Toxicity
Up to 60 % of patients treated with TKIs and mTOR inhibitors present with some form of skin toxicity including hand-foot syndrome (HFS), hair color changes, skin rash, dry skin, skin discoloration, nail changes, acral erythema, and subungual splinter hemorrhages. Skin toxicity in particular hand-foot syndrome (HFS) and skin rash has been described in up to 60 % of sorafenib-treated patients, approximately 30 % of sunitinib-treated patients, and less than 20 % in pazopanib-treated patients. Skin toxicity typically occurs after 2–4 weeks of treatment [48, 49]. HFS appears to be the most significant of these toxicities, while the other skin toxicities appear well manageable. Preexisting skin conditions should be evaluated and treated prior to TKI or mTOR therapy.
Hand-foot syndrome has been described with all TKIs but with varying frequency. Despite sharing the same spectrum of target receptors with sorafenib, axitinib, and sunitinib, pazopanib appears to be associated with an unexpectedly low risk of HFSR [50, 51].
Symptomatic HFS typically includes painful symmetrical erythematous and edematous areas on the palms and soles, commonly preceded or accompanied by paresthesias, tingling, or numbness. Desquamation can occur in severe cases as well as painful hyperkeratotic areas on pressure points surrounded by rings of erythematous and edematous lesions and painful bullous lesions, blisters, or skin cracking. Areas of pressure are particularly prone to these changes. Preexisting sole hyperkeratosis seems to confer a predisposition for painful sole involvement and functional consequences. TKI-induced HFS can interfere with function in severe cases. TKI-induced HFS is distinct from classic chemotherapy-induced HFS or palmar-plantar erythrodysesthesia.
The exact pathogenesis of this type of HFS is still unknown. Changes can been seen in the epidermal and dermal layers and followed throughout the course of HFS [52, 53]. The most consistent histologic changes are dermal vascular modifications with slight endothelial changes in grade 1–2 HFS and more pronounced vascular alterations with extensive and linear layers of keratinocyte necrosis and intraepidermal cleavage in grade 3 HFS and peribullous lesions [48, 54]. Unproven hypotheses regarding underlying mechanisms include inflammatory infiltration, secretion of the TKI into the eccrine glands resulting in direct toxicity to the skin, as is the case in doxorubicin-associated HFS, and dermal vessel alteration and endothelial cell apoptosis due to direct anti-VEGFR and/or anti-PDGFR [52, 54–57]. Blockade of VEGFR and PDGFR by sunitinib promotes tumor vessel regression by interfering with endothelial cell survival and repair mechanisms [58]. When endothelial survival mechanisms are inhibited in palmoplantar high-pressure areas subjected to repeated trauma through walking, hand washing, and other daily activities, such as the palms and soles, these areas may be unable to repair and thereby acquire the reactive characteristics of HFS [59, 60].
The dose-dependent relationship between TLKIs and HFS also suggests a direct toxic effect of TKIs in HFS pathogenesis [60]. Because an overlap in targets for sorafenib and sunitinib lies in VEGFR and PDGFR inhibition, HFS appears to be an indirect effect of the inhibition of these proangiogenic pathways [53, 60, 61]. The combined inhibition of these receptors appears to be essential because PDGFR (imatinib) or VEGF (bevacizumab) inhibition alone does not result in a similar rate of HFS [62].
Management strategies for HFS are preventative and symptomatic measures (Fig. 22.3). Preexisting calluses and hyperkeratotic areas should be removed prior to treatment. Moisturizers such as simple petroleum jelly-based ointments (e.g., Vaseline®, Aquaphor®) can be applied frequently right from the beginning of therapy. Foot and hand care products (e.g., gel pad inserts, cotton gloves, and clobetasol propionate cream) and medication for pain management can be used for symptomatic patients. Patients should decrease pressure on affected areas, staying off feet when possible and avoiding friction/pressure to hands. Shock-absorbing shoe insoles may be helpful to relieve painful pressure points as well as appropriate footwear and socks to draw moisture from the plantar surface [60]. Topical morphine can be used for patients experiencing severe pain. Steroid creams are also often used, although well-conducted studies are lacking. HFS is not an inflammatory response, but steroid creams may prevent secondary inflammatory processes from taking place. Topical skin adhesives (medical-grade super glue) applied to cracks and painful areas are an option.


Fig. 22.3
Recommendations for management of hand-foot syndrome
Treatment of ≥ grade 2 HFS usually includes the above-discussed measures but often requires dose interruptions, schedule alterations, and if necessary dose reductions as discussed previously. Grade 3 HFS almost always requires dose interruption and frequently subsequent reduction and/or schedule modifications.
For sunitinib therapy, schedule adjustments (e.g., 2 weeks on/1 week off) rather than dose adjustments are often useful as a first step since sunitinib-induced hand-foot syndrome tends to increase over the 4-week period and the pain generally improves quickly (usually within 2–3 days but may take 5 days or longer for higher grades) after removal of the drug. For other TKIs given continuously, brief (2–5 day) dose interruptions may provide substantial benefit while allowing for sustained long-term therapy.
If a patient believed to have HFS does not respond to dose interruption or dose reduction, then other diagnoses must be considered and if necessary treated, including fungal infection or overgrowth, dyshidrotic eczema, allergic contact dermatitis, and irritant dermatitis.
Generalized erythema and maculopapular or seborrheic dermatitis-like rashes have been reported in approximately 20–60 % of TKI-treated patients with the vast majority being NCI CTCAE grade 1–2 [1, 29, 48, 59, 63]. Skin rashes associated with TKI treatment rarely require dose reduction, and symptoms tend to decrease over time. Dose interruptions may be necessary for higher-grade skin rash (> grade 2), but patients usually can be rechallenged at the same dose level again after recovery to grade ≤1.
Patients should avoid hot showers, use sun protection, and wear loose-fitting cotton clothes. Moisturizing skin creams or lotions, e.g., a colloidal oatmeal lotion, should be frequently applied, in particular after showers and before bedtime [64]. Urea-containing lotions may be helpful, in particular if the skin is very dry. Anti-itch formulas and antidandruff shampoos can be used if itch or scalp discomfort is present [60]. Topical therapies, e.g., steroid creams, may be used for severe cases.
22.6 Oral Toxicity
Oral changes, including sensitivity and taste changes, dry mouth, as well as oral mucosal sensitivity (often referred to as stomatitis/mucositis), occur with varying frequency, in approximately 60 % of patients. Both tyrosine kinase inhibitors and mTOR inhibitors can cause mucositis, but most toxicities are ≤ NCI CTCAE grade 2.
Oral toxicities may occur as early as 7–14 days after the start of therapy. The oral reactions seen during treatment with targeted agents differ from those seen in chemotherapy-induced oral mucositis, which is characterized by local tissue damage and an inflammatory reaction and typically is associated with myelosuppression and mucositis throughout the gastrointestinal tract, causing diarrhea, nausea, and vomiting. TKI-induced oral toxicity in contrast appears to be primarily a “functional” irritation of the mucosa. Patients report a general sensitivity in the mouth which feels sore or they have alterations in taste, but clinical findings are largely normal, and patients do not experience the typical physical signs of a mucositis/stomatitis caused by chemotherapy (“functional stomatitis”). Although mouth ulcerations and aphthous stomatitis may be more frequently seen with mTOR inhibitors, almost all cases are low grade and manageable with supportive measures (grade 3/4 mTOR-associated stomatitis <5 %).
Very few data are available to describe the reactions seen with targeted agents, and the exact mechanism of targeted agent-induced oral toxicities remains unknown. VEGF has been found to be a component of normal human saliva, suggesting that salivary VEGF may play a role in regulating physiologic and pathologic angiogenic and other vascular responses in salivary and mucosal tissues [65].
Treatment for oral side effects is symptomatic only and consists mainly of a modified diet, nutritional consultation, and mouthwashes (Table 22.3). Good oral care should be maintained throughout TKI and mTOR therapy [66]. Oral toxicity can usually be managed symptomatically, and dose adjustments or treatment discontinuation is seldom necessary, while short treatment breaks can be advised for patients with significant discomfort.
Table 22.3
Recommendations for management of oral toxicities
Foods |
Avoid hot, spicy, or acidic foods |
Eat soft foods that are at room temperature |
Cut food into small pieces |
Use a straw for drinking liquids |
Oral care |
Perform routine home oral care |
Patients should be instructed to avoid alcohol-containing mouthwashes and consider using a children’s toothpaste if toothpaste causes burning |
Chlorhexidine and other antimicrobial agents are not warranted as there is no evidence to suggest that oral sensitivity is attributed to gingivitis or periodontal disease |
Symptomatic relief: |
Magic mouthwash containing equal parts of 2 % viscous lidocaine, diphenhydramine, and bismuth subsalicylate or aluminum/magnesium hydroxide |
22.7 Diarrhea
Diarrhea occurs in approximately 30–50 % of patients treated with TKIs, but grade 3/4 toxicity is rare and observed in only 3–8 % of cases. Some degree of diarrhea is often the main toxicity remaining when other common toxicities have been controlled with dose/schedule changes. In contrast to chemotherapy-induced diarrhea, which is usually continuous, TKI-induced diarrhea can occur irregularly with days of diarrhea mixed in with days of normal bowel movements. The incidence of diarrhea associated with mTOR inhibitors is lower (<20 %) with severe grade 3/4 diarrhea being very rare (1 %).
The underlying pathogenesis for TKI-induced diarrhea is not known. Bowel mucosa changes consistent with ischemic colitis have been reported after treatment with other VEGF-interacting agents, in particular bevacizumab [67].
Grade 1/2 diarrhea can usually be well managed by symptomatic measures including oral hydration, oral antidiarrheal agents as needed such as loperamide, and dietary changes. Patients can be advised to drink plenty of liquids (but in small amounts at a time, avoiding drinking fluids with meals and for 1 h after), eat and drink often in small amounts, and avoid spicy foods, fatty foods, caffeine, and high-fiber foods. Stool softeners and fiber supplements as well as magnesium-containing antacids should be discontinued.
Dose reductions are rarely necessary for grade 1 and 2 toxicity, while treatment should be interrupted for grade 3 or 4 diarrhea until diarrhea is grade ≤1 or has returned to baseline. Upon rechallenge, dose or schedule changes are frequently required to control diarrhea in subsequent cycles. Diarrhea usually resolves quickly during treatment breaks.
A number of other gastrointestinal side effects including taste changes, dry mouth, nausea, vomiting, and indigestion occur with varying frequency (10–30 %). Dose adjustments or interruptions are seldom necessary. Anorexia is found in about 10–20 % of patients but rarely exceeds grade 2. Although anorexia rarely requires dose modifications, underlying causes should always be investigated, in particular a potential relationship to coexisting hypothyroidism and other gastrointestinal toxicities. Patient education regarding nutrition and consultation with a dietician is recommended.
The emetogenic potential of TKIs and mTOR inhibitors is low. Less than 5 % of patients experience grade 3 or 4 vomiting/nausea and only 10–30 % grade 1–2 [1, 7, 68, 69]. Common antiemetics can be used to relieve or prevent nausea and vomiting. However, particular care should be used when combining targeted agents with antidopaminergic agents such as domperidone or 5HT3 antagonists, such as granisetron, ondansetron, and dolasetron, since they have been associated with QT/QTc interval prolongation and/or torsade de pointes, a potential side effect also associated with TKI therapy.
H2-blockers are recommended for the treatment of heartburn and indigestion.
22.8 Hematotoxicity
Myelosuppression has been observed with both TKIs and mTOR inhibitors. Sunitinib induces neutropenia and thrombocytopenia in about 20 % of non-Asian patients. Only 5–8 % of patients develop grade 3/4 neutropenia or thrombocytopenia, and very few cases of neutropenic fever have yet been reported. Treatment modifications should only be considered for grade 3 or 4 toxicity and/or clinical symptoms such as neutropenic fever or bleeding signs or for severe anemia. Blood counts usually recover quickly during treatment breaks. Hematopoietic growth factors are rarely required. Ethnic background appears to impact on the incidence of hematotoxicity. Recent data suggest a significantly higher incidence of myelotoxicity, in particular neutropenia and thrombocytopenia, in Asian patient populations [70].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree