Staphylococcal TSS is an acute and severe multisystem illness that was described initially by Todd et al. in 1978. The disease is characterized by sudden onset of high fever, hypotension, vomiting, diarrhea, erythematous cutaneous rash that desquamates during recovery, and multiple organ involvement. Although first described in association with
S. aureus in children, TSS occurs predominantly in adults, especially women. During the early 1980s, the overwhelming majority of staphylococcus TSS cases occurred in association with menstruation (MTSS). With the removal of high-absorbency and polyacrylate tampons from the market, TSS not associated with menstruation now occurs as frequently
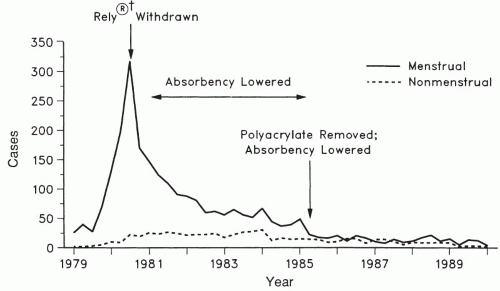
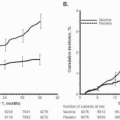
(Fig. 10.1). A unique
S. aureus toxin—toxic shock syndrome toxin 1 (TSST-1)—is associated with almost all menstruation-related TSS cases and at least half of nonmenstrual TSS (NMTSS) cases.
Epidemiology
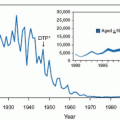
Shortly after Todd et al. introduced the term toxic shock syndrome and defined the clinical criteria that distinguished TSS, a precipitous increase in the number of TSS cases was reported in 1979 and early 1980. By April 1982, 1,654 cases of TSS had been reported to the Centers for Disease Control and Prevention (CDC). Between 90% and 95% of cases occurred in women, with the greatest risk in women younger than 30 years of age. The overwhelming majority (>95%) of menstruation-associated TSS cases occur in white women. Approximately 95% of TSS cases in women occurred in association with menstruation. Nearly 99% of women with menstruation-associated TSS wore tampons. At this peak in 1980, the estimated incidence of TSS ranged from three to 15 cases per 100,000 menstruating women per year. The sudden increase in TSS cases reported in 1980 may have been the result of several factors: (a) the introduction of new tampon products, (b) changes in the constituent materials of tampons, and (c) a change in S. aureus.
Since 1980, with increased public awareness in tampon habits and absorbency, the estimated incidence of TSS declined dramatically by 1990 to a level of two to four cases per 100,000 women per year (
Fig. 10.1). In 1980, there were 890 cases of TSS reported, of which 812 (91%) were associated with menstruation. By 1989, only 61 cases of TSS were reported, of which 45 (74%) were menstrual and by 2005 only 90 cases of TSS were reported to the CDC, with nearly 80% occurring in women.
Initial reports suggested that the case fatality rate for staphylococcal TSS was as high as 13%. Subsequent reports noted a dramatic decrease in the case fatality rate to approximately 3%. This improvement in survival most likely reflects increased awareness of TSS by clinicians resulting in early diagnosis and institution of appropriate therapeutic measures.
A dramatic decrease in the number of menstruation-associated TSS cases reported to the CDC was noted following the removal of Rely tampons from the market in September 1980 (
Fig. 10.1). In a review of MTSS from 1980 to 1990, the CDC suggested that the principal reason for the decreased incidence of MTSS may be the decreased absorbency of tampons. They noted that in 1980, very high-absorbency tampons (>15.4 g) were used by 42% of tampon users. In 1982, the United States Food and Drug Administration (FDA) required tampon package labeling advising women to use the lowest absorbency possible. As a result, by 1983 the proportion of very high-absorbency tampons had decreased to 18% and, by 1986, very high-absorbency tampons were used by only 1% of women using tampons. In March 1990, the FDA commenced standardized absorbency labeling of tampons ranging from
6 to 15 g. Very high-absorbency tampons are no longer marketed in the United States.
Tampon composition also is an independent risk factor for TSS and has changed since 1980. The Rely® tampon consisted of polyester foam and cross-linked carboxymethylcellulose, a combination no longer used in tampons. The unique composition of Rely tampons may have been responsible for the significant increased risk for MTSS associated with that product. Polyacrylate-containing tampons were withdrawn from the market in 1985 (
Fig. 10.1), resulting in a further decrease in reported cases of MTSS. Current tampons are manufactured from cotton and/or rayon.
Although reported cases of MTSS have declined substantially since the peak in 1980, reporting of NMTSS has remained constant during this time. Now nearly one half of TSS cases are nonmenstrual, up considerably from the 6% of TSS cases not associated with menstruation prior to 1981. Patients with TSS not associated with menstruation differ significantly in age and racial distribution from those with menstruation-associated TSS. First, it can occur in males, and TSS associated with infections not involving the vagina occurs equally in men and women. Of patients with non-menstruation-associated TSS, 11% were not white compared with only 2% of menstruation-associated TSS cases. The age range for non-menstruation-associated TSS ranged from the newborn to the elderly rather than the reproductive age span seen with menstruation-associated TSS.
It now is recognized that TSS is associated with a wide variety of conditions unrelated to menses and/or tampon use
(Table 10.2). The symptoms and signs in cases of NMTSS were similar to those described in menstruation-associated TSS, and
S. aureus was recovered from 88% of nonmenstrual cases. Nonmenstrual TSS has been reported in a variety of surgical procedures and soft tissue infection (
Table 10.2). TSS can occur in almost any situation in which
S. aureus infection can be harbored and an appropriate environment established to favor production of TSST-1. Disruption of normal skin or mucous membrane barrier that allows a localized
S. aureus infection to transmit toxins systemically appears to be a common factor in non-menstrual cases of TSS. TSS can occur as a complication of influenza and influenza-like illness especially with superimposed pneumonia.
A recurrence rate of approximately 30% was noted by investigators at the CDC. Multiple recurrences have been reported in the same patient. Recurrence rates are similar with MTSS and NMTSS. In both instances, recurrence was significantly more frequent in the absence of specific anti-staphylococcal therapy.
Etiology/Pathogenesis
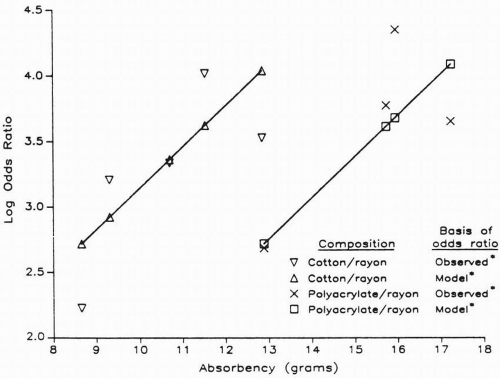
A significant association between staphylococcal TSS and tampon use was identified in a series of case-control epidemiologic studies in the early 1980s. While initial studies revealed no association between TSS and any specific brand of tampons, subsequent investigations demonstrated a significant association between TSS and the use of Rely® tampons. Investigators demonstrated that women who used high-absorbency tampons had a greater relative risk of developing TSS than women who used low-absorbency tampons. Clearly, tampon use has been established as a risk factor for TSS in menstruating women, with an odds ratio ranging from 11 to 18 in the initial studies conducted in 1980. The CDC compared tampon brands used by women with MTSS reported to the CDC passive surveillance system in 1983 and 1984 with those used by women in a national marketing survey during the same time period. The results demonstrated an increased risk with the use of tampons with increased absorbency
(Fig. 10.2). For each 1-g increase in absorbency, the risk of TSS increased by 37% (odds ratio [OR], 1.37; 95% confidence interval [CI], 1.29 to 1.45).
Several mechanisms by which tampons increase the risk of TSS have been suggested. Absorbency may be a surrogate for some other dose-response effect, such as increased oxygen content or increased ability to bind cations such as magnesium. Investigators raised a question whether the chemical composition of tampons might also be a factor n the development of TSS. The composition of materials used in tampons may play an important role in the development of TSS.
Prior to 1977, all tampon products were made of rayon or a rayon/cotton blend. After 1977, 44% of tampon products with 65% of the market contained more absorbent synthetic materials, such as polyacrylate fibers, carboxymethylcellulose, high-absorbency rayon-cellulose, and polyester foams. Neither cotton nor rayon tampons consistently increased toxin production by S. aureus, whereas tampons composed of carboxymethylcellulose and polyester foam increased production of staphylococcal exotoxins.
An association of TSS with
S. aureus was identified in the original case-control studies. Among TSS cases reported to the
CDC as of October 1982,
S. aureus was recovered from nearly all (210 of 215) patients.
The abrupt onset of the clinical presentation, the multisystem involvement, the rarity of bacteremia, and the similarity of TSS to other illnesses known to be caused by bacterial toxins led investigators to hypothesize that a toxin, either alone or synergistically with another factor(s), was responsible for TSS. With the demonstration that S. aureus was recovered from nearly 100% of TSS cases, attention focused on a staphylococcal toxin as the culprit.
It is now recognized that nearly all cases of menstrual TSS are caused by TSST-1, producing S. aureus. TSST-1 accounts for approximately 50% of nonmenstrual cases of TSS and staphylococcal enterotoxins-β and, to a lesser extent, SEC account for the remaining 50%.
Three factors have been suggested that account for the increased risk of menstrual TSS associated with high absorbency tampons. First, tampons increase vaginal oxygen potential and this increase results in greater production of toxin. Second, tampons that bind magnesium alter the growth kinetics of S. aureus, allowing greater production of TSST-1 during late logarithmic to early stationary phase. There is a direct correlation between absorbency and binding of magnesium, with the most absorptive fibers having the greatest capacity to bind magnesium. Third, some surfactants that are added to tampons enhance production of toxin.
TSST-1 has been shown to significantly enhance susceptibility to lethal endotoxin shock by 50,000-fold. It has been suggested that this enhancement of endotoxin explains the manifestations similar to Gram-negative septic shock that are associated with TSS, and that the combination of TSST-1 and small amounts of circulating endogenous endotoxin from the normal bowel flora provides an explanation for the pathogenesis of TSS. The massive release of IL-1 has been implicated in mediating many of the symptoms of TSS and TSST-1 is a potent stimulator of IL-1 release and is a more potent inducer of IL-1 than endotoxin. TSST-1 has also been shown to stimulate production of tumor necrosis factor alpha (TNF-α). Similarly, other TSS-associated exotoxins and endotoxins induce TNF production.
These findings are explained by the concept of super antigens (as described above), which are a class of bacterially and virally encoded proteins that stimulate a massive immune response by their ability to escape processing by antigen-processing cells and to bind directly with Class II major histocompatibility molecules, thus stimulating massive numbers of T cells. TSST-1 is such a superantigen.
The immunologic status, vis-à-vis TSST-1, may be important in the pathogenesis of TSS. Staphylococcal TSS patients have a greater serosusceptibility to TSST-1 than controls. Over 80% of sera from TSS patients had no detectable level of antibody, whereas nearly 80% of healthy controls had antibody levels ≥1:800. The sera from TSS patients also had lower levels of antibody to staphylococcal enterotoxins A, B, and C than did the controls. Thus, TSS patients may have an immunodeficiency that inhibits production of and/or maintenance of antibodies to TSST-1 and staphylococcal enterotoxins.
In summary, three steps are required for development of TSS. The patient must be colonized or infected with S. aureus. The staphylococci must be capable of producing TSST-1 and/or staphylococcal enterotoxin B and C. Last, there must be a portal for the toxin(s) to enter the systemic circulation. In NMTSS, S. aureus is present in a localized suppurative infection; as toxin is produced, it is absorbed from the infected site into the bloodstream. Several mechanisms by which staphylococcal toxin gains access to the systemic circulation in menstruation-associated TSS have been suggested. Toxin could be absorbed through microulcerations in the vaginal mucosa secondary to trauma from tampons and/or inserters. Superabsorbable tampons may obstruct menstrual flow, producing reflux of menstrual blood containing toxin through the fallopian tubes into the peritoneal cavity. This would result in absorption of toxin across the peritoneum. Alternatively, toxin could be absorbed across the denuded endometrial surface.
Clinical Presentation
TSS is a multisystem disease with a wide range of symptoms, signs, and laboratory findings. Characteristically, TSS occurs abruptly with the sudden onset of high fever, chills, myalgias, vomiting, diarrhea, hypotension, and generalized “sunburnlike” rash. The case definition, proposed by the CDC, is given in
Table 10.3.
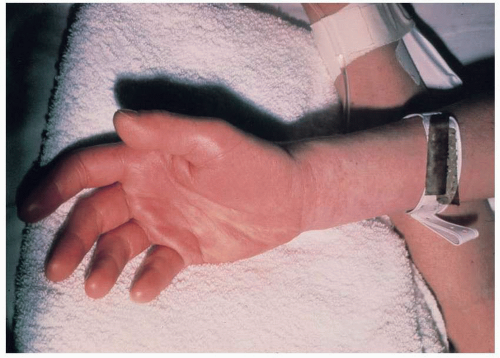
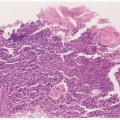
Involvement of the skin and mucous membranes is one of the most characteristic findings. The rash, which occurs early in the disease process, presents as a “sunburn-like” macular erythema; it can evolve to where the patient appears like a “broiled lobster”
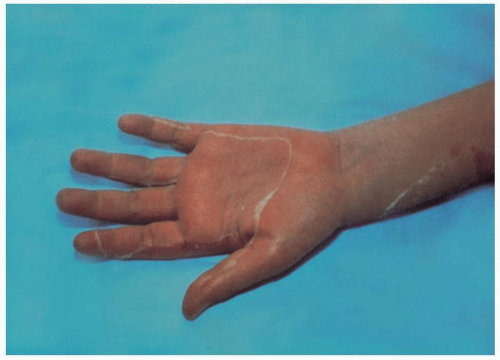
(Fig. 10.3). Five to 12 days after the onset of TSS in surviving patients, a fine desquamation occurs on the face, trunk, and extremities. This is followed by the nearly
pathognomonic full-thickness, peeling-like desquamation of the palms and/or soles
(Fig. 10.4). Staphylococcal toxin(s) has a predilection for mucous membranes, and their involvement is often a prominent feature of TSS. This manifests clinically as sore throat, oropharyngeal hyperemia, strawberry tongue, nonpurulent conjunctivitis, and/or vaginal hyperemia.
Gastrointestinal symptoms and signs are frequent and prominent in TSS. Vomiting and watery diarrhea have been noted in approximately 90% of cases. These symptoms occur early in the disease, usually are severe, and are associated with marked generalized abdominal tenderness.
Another early characteristic finding is the presence of myalgias, which have been reported in 88% to 100% of TSS cases. Exquisite muscle tenderness can be elicited by movement or touch.
Hypotension and shock are prominent features of TSS. They occur early in the disease process and constitute one of the major criteria for the diagnosis of TSS. The presence of orthostatic syncope now has been accepted as an alternative criterion for documented shock.
Renal involvement has been demonstrated in more than 80% of patients with TSS. This renal dysfunction has been manifested by sterile pyuria, mild proteinuria, hematuria, azotemia, hyponatremia, increased urinary sodium, hyperkalemia, and decreased creatinine clearance.
The prognosis for patients with TSS often is determined by the presence of pulmonary involvement, especially acute respiratory distress syndrome (ARDS) or “shock lung.” Tachypnea and hypoxemia (Po2<60 mm Hg) are seen frequently in TSS patients. Radiologic evidence of pulmonary edema and/or pleural effusions may be seen. Progression of these respiratory findings to ARDS in TSS patients is well documented. Most likely, ARDS occurs as a consequence of the enhanced activity of endotoxin caused by TSST-1. Once ARDS develops, the prognosis is poor, especially when extensive or prolonged ARDS is present.
The laboratory abnormalities reported in staphylococcal TSS cases reflect the multisystem involvement characteristic of the disease. Some of the more commonly reported metabolic and electrolyte abnormalities include metabolic acidosis, hypocalcemia, hypokalemia, hyponatremia, and hypophosphatemia. Among the frequent hematologic abnormalities are anemia, thrombocytopenia, leukocytosis, prolonged prothrombin time, increased prolonged partial thromboplastin time, and increased fibrin degradation products. Hepatic involvement is manifested by increased liver enzymes and hyperbilirubinemia. Renal involvement is signaled by the occurrence of azotemia, increased serum creatinine, pyuria, hematuria, and proteinuria. In addition, increased creatine phosphokinase connotes muscle involvement.
The distinctive pathophysiologic aspects of staphylococcal TSS include (i) the rapidity with which clinical manifestations can present and progress in a previously healthy individual; (ii) the rapid onset of hypotension secondary to decreased vasomotor tone and nonhydrostatic leakage of fluid to the interstitial space resulting in tissue ischemia and multisystem organ failure, (iii) the presence of multisystem organ involvement caused by direct action of toxin and/or mediators on cells and poor tissue perfusion; (iv) the spectrum of involvement of skin and mucous membranes; and (v) the high rate of recurrence in patients with untreated TSS.
Despite the severe clinical manifestations of TSS, the vast majority of patients recover without residual effects. The sequelae associated with TSS are divided into two groups. The early-onset findings occur between days 4 and 14 of the acute illness. This group includes the desquamation of skin and peeling of palms and/or soles; impaired sensation of the fingers; swollen, denuded tongue, transient vocal cord paralysis; acute tubular necrosis with renal failure; ARDS; and carpal tunnel syndrome. The second group of late findings occurs 60 or more days after onset of the TSS episode. Included in this group are splitting of the nails, reversible loss of hair and nails, CNS sequelae, renal impairment, cardiac dysfunction, and recurrent episodes of TSS. Of particular concern is the demonstration of persistent neurologic sequelae in survivors of TSS. In particular, abnormalities in intellectual function, such as impaired memory, concentration, and ability to perform mathematical calculations have been described.
It has been well documented that TSS can recur, especially in women with menstruation-associated disease. Recurrence rates from 28% to 64% were reported in the initial studies identifying TSS, with a recurrence rate of approximately 33% being the general consensus. Recurrences were significantly less frequent in patients who received β-lactamase-resistant, antistaphylococcal antibiotics during their initial episode of TSS. Because of confusion regarding what constitutes a recurrent episode of TSS, three categories based on the presence or absence of the major criteria for diagnosis of TSS have been proposed. These recurrence categories for TSS are given in
Table 10.4TSS associated with surgical wound infections generally presents as an early-onset wound infection within 48 h postoperatively. Unlike other early-onset wound infections caused by Clostridia perfringens or group A β-hemolytic streptococci, TSS-associated wound infections have minimal or absent local signs of infection such as erythema, fluctuance, or drainage. However, S. aureus is nearly always recovered from these benign-appearing wounds. The key clinical point is the occurrence of watery diarrhea and diffuse erythroderma in association with high fever in the initial several days postoperatively. Such findings, especially if hypotension is coexistent, must result in prompt evaluation of the operative wound for evidence of S. aureus infection.
Staphylococcal TSS is associated with a broad spectrum of clinical manifestations and many cases do not fulfill the strict epidemiologic criteria defined by the CDC, are generally milder, and do not present with life-threatening hypotension. As a result, a case definition of “probable TSS” has been promulgated
(Table 10.5). It is important for clinicians to recognize these milder cases of TSS to institute early and appropriate therapy. These milder “probable TSS” cases may subsequently develop into the severe, life-threatening form of TSS.