Fig. 20.1
Before (left) and after treatment with 5 % 5 FU, three times a week for 4 weeks
Askew et al. found in a review which systematically evaluated the topical use of 5-fluorouracil for the treatment of actinic keratosis in 29 studies that in general a complete clearance of lesions in half of the patients and a decrease between 80 and 90 % in the number of lesions should be expected; approximately 2/3 of patients require retreatment after 1 year, and although only a few patients discontinued treatment due to side effects, only half of them completed a full course of treatment. The authors felt that the level of evidence to evaluate the use of 5-fluorouracil in actinic keratosis was poor, taking into account the quality of the studies; also considered as poor is the level of evidence that exists for other therapeutic options available for treatment of actinic keratosis [11]. 0.5 % 5-fluorouracil cream preparations seem to have similar effectiveness when compared with the 5 % cream preparation in its ability to reduce the number of lesions, but their action depends more on the treatment time, which can be a problem because many patients stop treatment because of local adverse effects [12].
Actinic Cheilitis: Once a day for 7–14 days, a lot of swelling and inflammation is to be expected (kiss of fire); some authors recommend the use of topical corticosteroids after the treatment to help resolve the inflammation. Epstein reported good experience in 12 patients with recurrences in only 2 patients; there was inflammation with cracking and scabbing, but they report a good cosmetic result [13].
Bowenoid Papulosis: 1–2 daily applications for 4–6 weeks. This treatment should be considered if surgery is not possible [14].
Patients treated with 5-fluorouracil usually develop scaling, erythema with vesicles and blisters formation, pain, and burning, and patients should be warned in advance. Sometimes the frequency of drug application must be decreased to a daily and even a one another day schema depending on the severity of the reaction. The process of re-epithelialization and healing takes place between 1 and 2 months [5, 8].
5-Fluorouracil has been used systemically for the treatment of metastatic breast and gastrointestinal carcinomas as well as ovary, cervix, prostate, bladder, pancreatic, and pharyngeal carcinomas [4].
Available Forms: 0.5, 1, 2, and 5 % cream and lotion. Most reports of topical treatment of skin cancer have been with the 5 % cream.
Safety in Pregnancy (See Table 20.1 for Category Definitions): Category D (IV), Category X (topical).
Table 20.1
United States Food and Drug Administration (FDA) pregnancy categories
Category | Description |
|---|---|
A | Controlled studies show no risk – adequate, well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester of pregnancy |
B | No evidence of risk in humans – adequate, well-controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility |
C | Risk cannot be ruled out – adequate, well-controlled human studies are lacking, and animal studies have shown a risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is administered during pregnancy, but the potential benefits may outweigh the potential risk |
D | Positive evidence of risk – studies in humans or investigational or post-marketing data have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective |
X | Contraindicated in pregnancy – studies in animals or humans or investigational or post-marketing reports have demonstrated positive evidence of fetal abnormalities or risk which clearly outweigh any possible benefit to the patient |
Adverse Effects: Erythema, peeling, blistering, contact dermatitis, photosensitivity, phototoxicity, burning sensation, angioedema, erythema multiforme, rash, folliculitis, necrosis, pigmentation, pruritus, seborrheic dermatitis, lupus-like syndrome, xerosis, alopecia, onycholysis, nail pigmentation, dysgeusia, ectropion, mucositis, paresthesia, stomatitis, and tongue pigmentation [15].
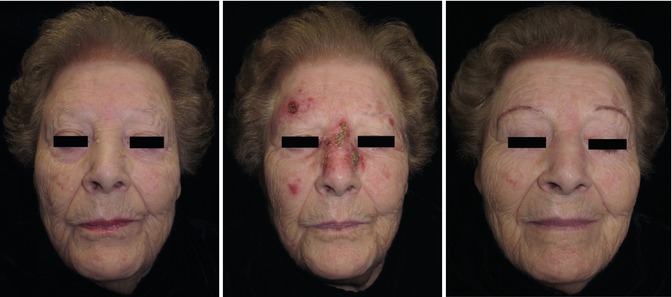

Fig. 20.2
Pretreatment (extreme left), immediately posttreatment, and after 1 month posttreatment with imiquimod for three weeks
Imiquimod
Imiquimod is an imidazoquinoline amide which acts by stimulating the immune response both innate and acquired. It has antiviral, antitumor, and immunoregulatory activity [3, 5].
Pharmacology: It works by stimulating toll-like receptor 7 on dendritic cells and induces the production of various cytokines such as tumor necrosis factor alpha, interferon alpha, and interleukins 1, 6, 8, and 12. Interferon alpha produced by a dendritic cell interacts with the FAS-FAS ligand complex of the tumor cell, leading to apoptosis. Interleukin-12 induces production of interferon gamma by CD4 lymphocytes activating cytotoxic T lymphocytes to act directly against tumor cells [3, 5].
Formula: C14H16N4
Half–Life Time: 30 h (topical dose), 2 h (subcutaneous dose)
Uses:
Squamous cell carcinoma in situ (Bowen’s disease)
Actinic cheilitis
Basal cell carcinomas with a low risk of recurrence or in patients who are not able or willing to undergo surgical treatment
Actinic keratosis (Fig. 20.2)
Cutaneous T-cell lymphoma (mycosis fun-goides)
Malignant melanoma in situ, in patients that are not candidates for surgical treatment
Metastatic malignant melanoma as an adjunct therapy to chemotherapy and as a palliative treatment that helps to control local skin metastases
Dosage:
Squamous Cell Carcinoma In Situ (Bowen’s disease): 1 application per day for 9–16 weeks
Mackenzie-Wood et al. in 2001 reported clinical and histopathologic cure in 15 of 16 patients treated: there was no relapse or recurrence in a follow-up period of 6 months. This effectiveness has been confirmed by other case reports [16–18].
Superficial Basal Cell Carcinoma:
2 daily applications for 12 weeks (effectiveness of 100 %) [19]
1 daily application for 12 weeks (effectiveness of 87 %) [19]
1 daily application, 5 times a week for 12 weeks (effectiveness of 81 %) [19]
1 daily application, 3 times a week for 12 weeks (effectiveness of 52 %) [19]
2 daily applications for 6 weeks (effectiveness of 100 %) [20]
1 daily application for 6 weeks (effectiveness of 88 %) [20]
2 daily applications, 3 times a week for 6 weeks (effectiveness of 73 %) [20]
1 daily application, 3 times a week for 6 weeks (effectiveness of 67 %) [20]
Nodular Basal Cell Carcinoma:
1 daily application for 12 weeks (effectiveness of 78 %) [21]
1 daily application, 5 times a week for 12 weeks (effectiveness of 70 %) [21]
1 daily application, 3 times a week for 12 weeks (effectiveness of 60 %) [21]
1 daily application for 6 weeks (effectiveness of 71 %) [21]
2 daily applications, 3 times a week for 6 weeks (effectiveness of 42 %) [21]
1 daily application, 3 times a week for 6 weeks (effectiveness of 59 %) [21]
In basal cell carcinoma, the dosing regimen and tumor histopathologic variety seem to directly influence the effectiveness of treatment. The higher the frequency of daily and weekly application, the higher is the effectiveness. It is more effective in the superficial histopathologic variety than in the nodular variety and is more effective in lowering the risk of recurrence in anatomic locations such as trunk and limbs. The problem is that the higher the frequency of application, the higher are also the side effects like inflammation, crusting, edema, and pain. These side effects can significantly affect adherence to treatment [20–22].
Geisse et al. reported an 82 % cure rate in treating small superficial basal cell carcinomas with a 5 times a week for 12 weeks course of treatment and 79 % cure rate in a 7 times per week for 12 weeks scheme compared with the vehicle results that were close to 2 % [22].
Schulze et al. reported an 80 % histopathologic cure rate in treating superficial subtypes using a 1 application a day for 7 days a week for 6 weeks schema; the cure rate with the vehicle was 6 % [23].
Gollnick et al. conducted a prospective study in 182 patients with superficial basal cell carcinomas treated with 5 % imiquimod cream, 5 times per week for 6 weeks, resulting in a cure rate of 69 % at 5 years; apparently the effectiveness of treatment decreases the longer the follow-up periods are [24].
Eigentler et al. reported a clinical and histopathologic response of 72 and 64 %, respectively, in a phase 3 study that evaluated the effectiveness of imiquimod 5 % cream for the treatment of nodular basal cell carcinoma in 102 patients using a regimen of one application 3 times a week for 8 and 12 weeks [25].
Edwards et al. reported a 75 % reduction in the number of actinic keratosis using imiquimod 5 % cream 3 times a week for 16 weeks in 40 patients [26].
Salasche et al. conducted a study in 25 patients that used imiquimod 5 % cream, 3 applications per week for 4 weeks followed by a 4-week rest period; if at that time lesions persisted, another course of 4 weeks of treatment was indicated, with a maximum of 3 cycles (12 weeks of treatment and 12 off). Complete response in 82 % of lesions was obtained including subclinical lesions that become evident with treatment; this is a good way to maximize the effectiveness of treatment by decreasing adverse effects [27].
A similar scheme was tested in a phase 3 study conducted by Stockfleth et al. in 829 patients using 2 cycles of 3 times a week for 4 weeks schema, getting total clearance of lesions in 68.9 % and partial clearance in 80.2 % [28].
In a randomized, multicenter, double-blind, placebo study conducted by Szeimies et al. in 286 patients, the use of imiquimod 5 % cream 3 times per week for 16 weeks compared with placebo cream was evaluated; complete response was observed in 57.1 % of lesions in subjects treated with imiquimod compared with 2.2 % in those patients treated with placebo [29].
In a meta-analysis conducted by Gupta et al., imiquimod 5 % cream and 5-fluorouracil 5 % cream were compared for topical treatment of actinic keratosis on the face and scalp, and they concluded that imiquimod was more effective than 5-fluorouracil (70 ± 12 % vs. 52 ± 18 %, respectively) [30].
Actinic Cheilitis: 1 application 3 times a week for 4–6 weeks.
Smith et al. reported total clinical response in 100 % of the 15 patients with diagnosis of actinic cheilitis treated with imiquimod; the result was maintained for 4 weeks of follow-up. No histopathologic control was made. Use of oral valacyclovir as a preventive measure in patients with a personal history of herpes in the facial region was recommended (1 g/day) [31].
Lentigo Maligna: 1–2 applications per day for 5–7 days a week. Treatment time varies depending on the series (12 weeks to 1 year).
Ahmed et al. reported its use for the first time in lentigo maligna in 2000 in an elderly patient who refused to undergo surgery; they used it for a total period of 7 months with frequent rest periods due to local inflammatory reactions and ulcerations and found no evidence of recurrence or relapse 9 months later [32].
Naylor et al. reported histopathologic cure in 26 of 28 (93 %) patients treated with a regimen of one application per day for 12 weeks and no recurrences or relapses during a follow-up period of 1 year [33].
Van Meurs et al. reported in 2010 a 100 % histopathologic cure rate in ten patients with lentigo maligna; the average follow-up was 36 months [34].
Imiquimod is recommended in patients who are not candidates for surgery, which remains the treatment of choice, treatment should be prolonged, and a close and long follow-up period is advised. Cure rates ranging from 60 to 100 % have been reported. Effectiveness of topical imiquimod treatment is not well established in cases of invasive lentigo malignant melanomas and is not recommended due to the risk of local, regional, and distant organ metastases [33, 35].
Narayan et al. reported in a small pilot study an increased number of T cells in local and regional skin and in sentinel lymph nodes in patients with primary invasive malignant melanoma treated with imiquimod; further studies are needed in this type of tumor to clarify the meaning of these findings [36].
Metastatic Malignant Melanoma: 3–5 times a week and extends to periods that vary depending on the series 10–28 weeks. Treatment regimens vary and have been used alone and in combination with dacarbazine, interleukin-2, interferon alpha-2b, and 5-fluorouracil. Most authors report its use under occlusion. Good response rates for local disease control were reported [37, 38].
Available Forms: 3.75 and 5 % cream
Safety in Pregnancy: Category C
Adverse Effects: Inflammation can become severe, crusting, blistering, flu-like malaise, lupus-like reactions, psoriasis, edema, erosions, erythema, abrasions, fungal infections, pigmentation, itching, pain, ulceration pigmentation of hair, aphthous stomatitis, depression, induration, and myalgias [4, 15].
Mechlorethamine Hydrochloride (Nitrogen Mustard)
Is an alkylating agent that acts mainly through alkylation of DNA, generating a cytotoxic effect [3, 4].
Pharmacology: The most important pharmacological effect is that it interferes with DNA synthesis and cell division. The capacity of alkylating agents to affect the function and integrity of DNA in tissues that reproduce quickly and fast is what gives this drug its clinical utility and is also what accounts for their toxicity when used systemically. The cytotoxicity of these agents is enhanced in proliferating tissues in which most cells are actively dividing and their action is even greater in those cells with DNA damage and that are in the process of actively dividing. If the cellular mechanisms of DNA repair in cells are intact and are able to repair target DNA before the next division, the effect of these agents is not lethal. The seventh nitrogen atom of guanine is the most likely position where covalent bonding with the alkylating agent occurs. This also applies to other atoms of the purine and pyrimidine bases in DNA, such as the first and third nitrogen atoms of adenine, the third nitrogen atom of cytosine, and the sixth oxygen atom of guanine. It is a very unstable drug that is rapidly degraded. It can only be used intravenously and topically. It undergoes rapid chemical transformation on contact with water and tissues. Within minutes after administration, it is not possible to detect the drug in its active form [3, 4].
Formula: C5H11Cl2N
Metabolism: Rapid plasma hydrolysis, demethylation in plasma
Half–Life Time: <1 min
Excretion: Urine (50 % as metabolites, <0.01 % as unchanged drug)
Uses:
Cutaneous T-cell lymphoma (mycosis fungoides) in stages IA and IB. Therapeutic schemes usually start with daily applications over the entire affected area, decreasing the dosage to one another day application after winning control of the disease, depending on patient response and severity of local adverse effects. Two types of preparations have been used, the aqueous formulation for which a solution is prepared at a concentration of 10 mg/50 ml; this solution is applied with a brush on the affected areas and must be prepared daily, and this drug preparation is rapidly degraded on contact with water. The other way to prepare it is by using petrolatum as a vehicle in a concentration of 10 mg per 50 g of petrolatum; this has the advantage of producing less irritation and being more stable, does not require daily preparation, and should be applied with hand protected by latex or rubber gloves. Some authors recommend concomitant treatment with high-potency topical steroids, which reduces or attenuates the contact dermatitis caused by treatment and enhances the antineoplastic effect.
Treatment should be extended until 2–3 years after disease control is achieved.
Nondermatological Uses: Hodgkin’s disease (stages III and IV), lymphosarcoma, acute myelocytic or chronic lymphocytic leukemia, polycythemia vera, and bronchogenic carcinoma
Dosage
Mycosis Fungoides: Start with everyday applications and then decrease the frequency of application according to the therapeutic response and side effects. Treatment should be extended until 2–3 years after disease control is achieved.
de Quatrebarbes et al. reported 61 % of complete response in patients with stage IA mycosis fungoides, 58 % of complete response in patients with stage IB, and 40 % of complete response in patients with stage IIA, after a treatment period of 3,6 + 2,5 months. Twenty-eight percent of patients discontinued treatment due to local adverse effects, and 46 % of patients relapsed after a disease-free interval of 7.7 ± 6.5 months [39]. Others have reported complete response rates of 76–80 % for stage IA and 35 to 68 % for stage IB patients. Response rates are similar for both preparations used (aqueous and ointment) [40].
Available Forms: 10 mg mechlorethamine hydrochloride powder vial
Safety in Pregnancy: Category D. May cause fetal harm if the drug is absorbed during pregnancy.
Adverse Effects: Delayed hypersensitivity reactions (contact dermatitis), hyperpigmentation, hypopigmentation, other skin malignancies (spindle cell carcinoma), angioedema, herpes zoster, erythema multiforme, pruritus, purpura, xerosis, rash, alopecia, anaphylactoid reactions, dysgeusia, and tinnitus [4, 15].
Carmustine
Is an alkylating agent of the nitrosoureas group [4].
Pharmacology: Its therapeutic effect is done through DNA alkylation at the O6 position of guanine. Cell death occurs in all phases of the cell cycle. It is unstable in aqueous solutions and body fluids. The half-life ranges from 15 to 90 min and its excretion occurs in urine, 80 % as degradation by-products 24 h later [4].
Formula: C5H9Cl2N3O2
Bioavailability: 5–28 % (oral)
Protein Binding: 80 %
Metabolism: Hepatic
Half–Life Time: 15–30 min
Uses:
Cutaneous T-cell lymphoma (mycosis fungoides). It is the option of choice in patients allergic to mechlorethamine. Therapeutic schemes usually start with one another day applications over the entire affected area, space dosages depending on patient response and severity of local and systemic adverse effects. Two types of preparations, the aqueous formulation and petrolatum ointment, have been used.
Dosage: 10 mg once daily for 7–14 weeks (maximum 17 weeks). If the response is poor, give a second course of topical treatment with 20 mg once a day for 4–8 weeks according to tolerance after a rest period of 6 weeks.
It has been reported a partial or a complete remission in 92 % of stage IA patients and in 64 % of stage IB patients treated with carmustine after a follow-up period of 36 months [41, 42].
Available Forms: Vials of 100 mg powder
Safety in Pregnancy: Category D. May cause fetal harm if the drug is absorbed during pregnancy
Adverse Effects: Dermatitis in up to 50 % of cases, telangiectasias, erythema, bone marrow suppression, eccrine syringometaplasia, rash, flushing, cutaneous pain, alopecia, gynecomastia, oral mucositis, and stomatitis. It is recommended to control blood cells in lab tests. It has been reported an increased risk of pulmonary fibrosis when combined use with bleomycin or radiotherapy [4, 15].
Alitretinoin (9-cis Retinoic Acid)
It is a first-generation retinoid that has been used to treat HIV-associated Kaposi’s sarcoma.
Pharmacology: It binds to and activates all substrates of intracellular retinoid receptors. Once these receptors are activated, they act as transcription factors that regulate the expression of genes that control the differentiation and proliferation of both normal and neoplastic cells.
Formula: C20H28O2
Uses:
Topical: Treatment of Kaposi’s sarcoma lesions associated with AIDS should not be used when systemic treatment for Kaposi’s sarcoma is indicated.
Oral: Chronic eczema of the hands in patients who have not responded to conventional therapy.
Dosage:
Topical: 2–4 applications daily for 12 weeks
Response rates of 35–37 % in patients with epidemic Kaposi’s sarcoma have been reported; most were partial responses [43]. There is anecdotal evidence on case reports that it is less effective for the treatment of classic Kaposi’s sarcoma [44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree