Fig. 8.1
Clinical presentation of a DFSP grown in the region of the trunk
Diagnosis of Dermatofibrosarcoma Protuberans
Diagnosis is made using incisional biopsy [11]. An excisional biopsy is usually performed in all cases where it is possible to remove the entire lesion.
Magnetic resonance imaging (MRI) can be used to evaluate the local extent of the tumor.
Histopathology
DFSP has a characteristic histologic appearance of a poorly circumscribed uniform population of bland spindle cells arranged in a monomorphous storiform or “herringbone” pattern (Fig. 8.2a, b), which extends into the subcutis, often with infiltration into and/or around the adipose tissue. The epidermis over the lesion is usually normal or atrophic. Early lesions may demonstrate a “grenz zone,” which is a tumor-free region separating the tumor from the epidermis. Cutaneous adnexa are often found entrapped within the tumors. Mitotic activity usually averages fewer than 5 mitotic figures per high-power field. Unusual variants of DFSP include the Bednar tumor that is characterized by melanin-containing cells [10] the myxoid DFSP that has deposits of interstitial mucin, and giant cell, and the atrophic type.
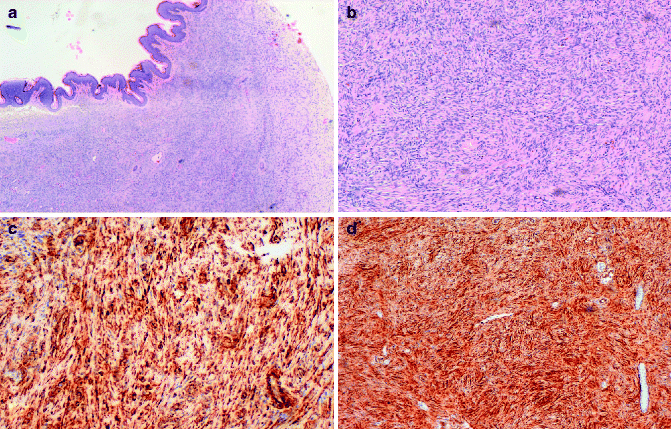

Fig. 8.2
(a) DFSP composed of slender fibroblasts arranged in a storiform pattern; (b) higher magnification, better showing a poorly circumscribed uniform population of bland spindle cells arranged in a monomorphous storiform pattern; (c) the neoplastic cells express CD34; (d) the neoplastic cells are positive for vimentin
In contrast, the fibrosarcoma is characterized by long fascicles of spindle cells with more evident nuclear atypia and higher mitotic activity. Approximately 15 % of cases of DFSP contain a component of high-grade sarcoma. Immunohistochemical analysis can be utilized for the diagnosis. DFSPs are positive for vimentin, focally and inconstantly for actin, and strongly and consistently for CD34 (Fig. 8.2c, d). Staining for CD34, indeed, is commonly employed, and sensitivity has been reported as being between 84 and 100 % [11, 12]. Positivity for CD34 is lost within the areas of sarcomatous change. Conversely, they are negative for S100 protein, HMB45, FXIIIa, and CD44.
Differential Diagnosis
Differential diagnosis with neurofibroma can be challenging since both DFSP and neurofibroma display CD34-positive cells, even if they are more abundant in DFSP; on the other hand, S100 protein is positive in neurofibromas and negative in DFSP. Concerning the differential diagnosis with dermatofibroma, the most important marker to be considered is CD34, since DFSP is consistently positive for CD34, while dermatofibroma lacks CD34-positive cells except for blood vessel endothelium.
Genetics
Most (90 %) of DFSP exhibit a chromosomal translocation between chromosomes 17 and 22. Consequence of this translocation is a fusion between the platelet-derived growth factor-B gene (PDGFB; chromosome 22) and the collagen 1 alpha 1 gene (COL1A1; chromosome 17), that leads to IPER-expression of the fusion oncogene and fully functional PDGFB [13, 14]. Detection of gene fusion by RT-PCR or FISH is helpful for confirming a diagnosis of DFSP.
Prognosis
DFSP is a locally aggressive tumor and despite sharing some histologic features with fibrohistiocytic tumors, it tends to grow in a more infiltrative fashion and has a greater capacity for local recurrence. Moreover, in rare instances, it gives distant metastases, but this is usually a late event.
Staging of Dermatofibrosarcoma Protuberans
DFSP is currently staged in accordance with the American Musculoskeletal Tumor Society Staging System which considers tumor grade and compartmentalization [15].
Treatment of Dermatofibrosarcoma Protuberans
The mainstay of treatment of DFSP is surgery [16]. In cases of very large or advanced DFSP, a multidisciplinary approach is recommended. Recent NCCN guidelines recommend margins of 2–4 cm using conventional surgical management. However, the use of Mohs surgery, allows complete excision with microscopic margins [17].
Merkel Cell Carcinoma
Merkel cells are normal constituent of the basal layer of the epidermis and the follicular epithelium and are thought to act as mechanoreceptors [18]. The Merkel cell carcinoma (MCC) was first described in 1972 as a sweat gland carcinoma by Toker [19]. Successively, the same investigator by means of electron microscopic studies identified dense-core neuroendocrine granules within the tumor cells, thus demonstrating their origin from Merkel cells [20]. The histogenesis of MCC, however, is still a debate, since certain differences exist between Merkel cells and MCC in terms of anatomical localization, of expression of neurofilaments, and of mitotic activity. Nevertheless, starting from the work of Johannessen in 1980, the term “Merkel cell carcinoma” was established [21].
Epidemiology
Clinical Features
MCC most commonly occurs as a solitary nodule in the elderly (Fig. 8.3), usually in sun-damaged skin, being the head and the neck region the most common site [24]. UV irradiation is recognized as one of the most important pathogenetic factors [25]. The clinical presentation of this tumor is rather nonspecific and especially small tumors can appear somewhat benign. Indeed, it is often mistaken for more common skin tumors of epithelial origin. MCC consists of nodular, firm, red-pink, non-ulcerated lesion, ranging from 1 to 4 cm in diameter. The natural history of MCC is variable, but typically tends to progress rapidly, with early and frequent metastasis to the regional lymph nodes. The overall survival rate is significant influenced by tumor size (≥2) [26]. Recurrences are frequent; however, there are some reports in the literature of spontaneous regression [27].
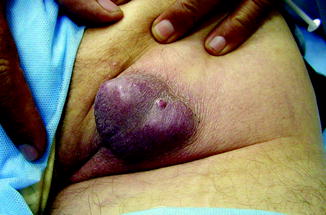

Fig. 8.3
Clinical presentation of a MCC grown in the perianal region (Dr Miguel López, Venezuela, personal observation)
Diagnosis of Merkel Cell Carcinoma
The clinical presentation of this tumor is generally nonspecific; therefore, diagnosis of MCC is essentially based on peculiar histology representation on hematoxylin-eosin-stained slides together with the data from immunohistochemistry. Electron microscopy studies can confirm the presence of neuroendocrine granules in the tumor cells, as it is the case for normal Merkel cells.
Histopathology
MCC typically consists of small round blue cells. The tumor cells are monomorphous, the cytoplasm is usually scant, and the nuclei are relatively uniform with chromatin pattern finely granular (Fig. 8.4a). Histologically, MCC can be classified into three distinct subtypes: trabecular, intermediate, and small-cell type [28]. Trabecular is the least frequent type; tumor cells are organized in organoid clusters and trabeculae. In the intermediate subtype, the most common histologic subtype, there is a solid and diffuse growth pattern. The small-cell subtype is similar to small-cell tumors of other site, such as small-cell lung cancer. Microscopically, MCC is located in the dermis or sometimes in the subcutaneous tissue, with the epidermis usually uninvolved and with a thin grenz zone. Ultrastructurally, the tumor cells contain dense-core neurosecretory granules and tightly packed perinuclear intermediate filaments.
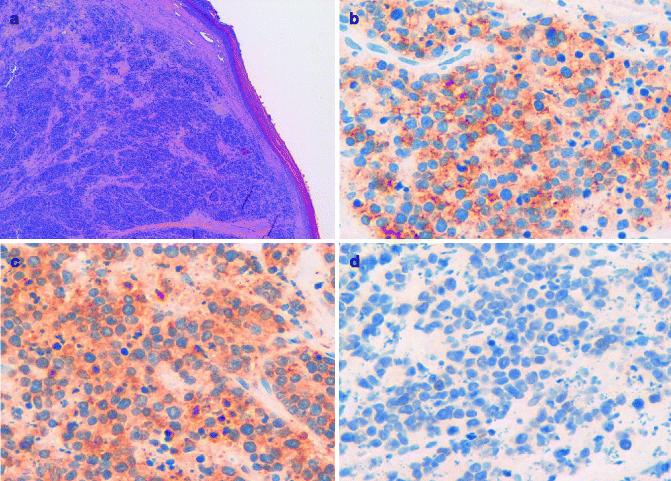

Fig. 8.4
(a) MCC composed of small round monomorphous blue cells centered in the dermis, with the overlying epidermis uninvolved; (b) the neoplastic cells express Cytokeratin 20; (c) the neoplastic cells are positive for synaptophysin; (d) the neoplastic cells do not express TTF-1
Immunohistochemistry can aid in the histological diagnosis of MCC, demonstrating the expression of both epithelial and neuroendocrine markers. Among the epithelial markers, the most important is cytokeratin-20, which is normally expressed only in the gastrointestinal epithelium, urothelium, and Merkel cells (Fig. 8.4b) [29]. Due to its neuroendocrine differentiation, MCC always stains positive for different neuroendocrine markers, such as neuron-specific enolase, chromogranin A, synaptophysin, and CD56 (Fig. 8.4c).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








