Author
Bernard [10]
Dessinotti [22]
Year/country
2008/France
2011/Greece
N
1,655
199
Head/neck
64.5 %
78 %
Trunk
25.1 %
15 %
Limbs
10.4 %
7 %
BCC is rarely described in black, Oriental and Hispanic people [16, 17]. In particular black people have a lower incidence of BCC in sun- exposed areas, but in unexposed skin regions, the incidence is similar to that observed in white people, being most of them of pigmented BCC subtype (>50 % of pigmented lesion as compared with 6 % in Caucasians) [7, 16, 18, 19].
Aetiology
The aetiology of BCC is still unclear, but its development seems to be related to combined contribution of endogenous and exogenous risk factors. Individual risk factors for BCC include gender, age, immunosuppression, genetic dysfunctions and pigmentary trait. Among exogenous risk factors, ultraviolet radiations (UVR) represent the primary established risk factor in BCC pathogenesis [20–23]. The relation between UV radiations and BCC development seems complex and remains highly controversial, with regard to the pattern of sun exposure and their occurrence in different period in life [18]. UVB radiation generates mutagenic photoproducts in DNA, such as cyclodipyrimidine dimers, and then mutations in specific genes related to critical cell functions, such as the p53 tumour suppressor gene. UVA rays have an indirect effect by generating cytotoxic and mutagenic free radicals, favouring the effect of UVB rays [20]. The mutagenic effects are directed also on critical genes, as p53 and PTCH1 [20, 22, 23]. Finally, UV rays have an indirect immunosuppressive action on the skin, harming the local antitumour monitoring activity of dendritic cell [12, 13]. Sun exposure, as predisposing factors, is particularly evident in patient with xeroderma pigmentosum, characterised by occurrence of multiple basal cell carcinoma and squamous cell carcinoma [12]. It has been also shown that occupational cumulative sun exposure is associated with BCC development in the older age group, whereas the prevalence of individuals with acute recreational sun exposure particularly in childhood and adolescence was significantly higher in the youngest age group of BCC [1, 24]. Prospective studies showed increased BCC risk with the number of sunburns after the age of 20 years old [10].
Additional factors favouring the development of both basal cell carcinoma and squamous cell carcinoma are large or numerous doses of roentgen rays, radiotherapy treatment, phototherapy (PUVA or UVB) and arsenic exposure [6, 16, 25–27] and, less commonly, burn scars and other scars. The role of dark hair dye colours, tobacco, alcohol, diets rich in fat and artificial tanning and photosensitising drugs in promoting BCC onset needs to be further clarified [26]. Thus, exposure to paraffin, coal, tar, pitch, industrial oils, agricultural chemicals, pesticides, ionising radiation and tattoos has been sporadically reported [24, 27–30]. The consumption of high daily doses of coffee (>6 cups) is associated with a reduction of up to 30 % in the prevalence of non-melanoma skin cancer in Caucasian women. Indeed, caffeine showed a photoprotective effect and reduced UVB-induced carcinogenesis by inducing apoptosis in the skin of mice. The same was observed in culture keratinocytes, in which caffeine increased the rate of apoptosis by inhibiting the ATR-Chk1 pathway [31–34].
The occurrence of consecutive BCCs is common, and the recurrence is more common in the first year. In 3 years the risk of a BCC patient to develop a further BCC ranges from 27 to 44 %, reaching 50 % in 5 years. Patients with more than ten BCCs have more than 90 % chance to develop a new lesion. Male patient older than 60 years, trunk localisation, superficial histological subtype and the presence of multiple actinic keratoses are predictive factors for the occurrence of new lesions [1, 5, 35, 36].
Pathogenesis
The Hedgehog Signalling Pathway in Gorlin-Goltz Syndrome: A Model of BCC Development
The Hedgehog signalling pathway is a well- conserved developmental pathway from insects to mammals [37]. Three homologues have been identified in humans, including the Sonic Hedgehog homologue (SHH), critical for the embryonic development and maintenance of the nervous system, axial skeleton, lungs, skin and hair. SHH is the ligand of a transmembrane receptor named PTCH1. The mechanism through which PTCH1 inhibits SHH signalling is complex [37]. PTCH1 represses the activity of the receptor Smoothened (SMO), which normally activates a family of transcription factors, termed glioma-associated transcription factors. Thus, the SMO protein, when unbound by PTCH, acts as a signal transducer, upregulating the expression of glioblastoma (GLI)1 and GLI2 signalling proteins, that eventuate in cell proliferation [38]. In addition Gli proteins control the expression of PTCH1, which provides a negative feedback of Hedgehog signalling, and GLI1 itself, which operates a positive feedback of Hedgehog ligand, thus regulating how much SHH is available to bind PTCH [39]. Upregulation of Gli proteins in BCC may play an important role in the initiation of BCC through the activation of the Bcl-2 gene [40, 41]. In addition GLI2 induces G1–S phase progression in contact-inhibited keratinocytes, apparently through the transcription factor E2F1 [42] (Fig. 5.1).
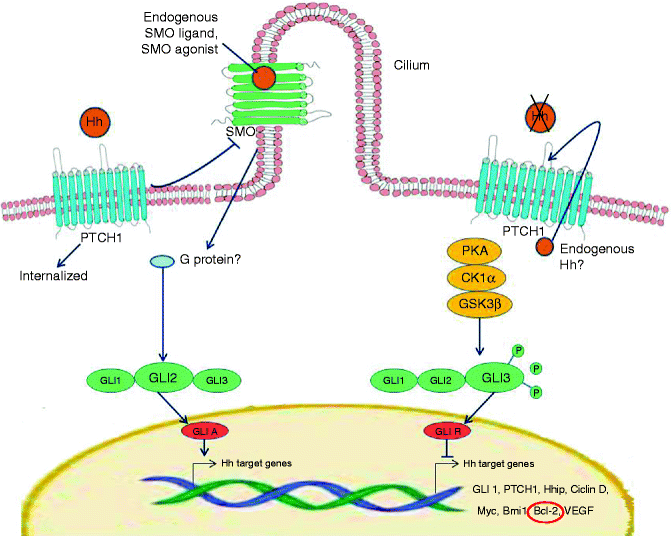

Fig. 5.1
Hedgehog pathway deregulation in BCC. Hedgehog signal transduction is induced by the patched 1 protein (PTCH) activated by the SHH ligand. Thus, binding of SHH to PTCH causes the loss of PTCH activity and the consequent activation of SMO, which transduces the SHH signal to the cytoplasm. Indeed, SHH signal is transmitted via GLI transcription factors, three separate zinc-finger proteins, GLI1 and GLI2 functioning as transcriptional activators and GLI3 as transcriptional repressor. SHH signalling cascade promotes generation of the activator GLIA, largely derived from GLI2, and the subsequent expression of a series of genes, especially Bcl-2. In the absence of a SHH ligand, PTCH blocks SMO activity, and full-length GLI proteins are processed to generate the repressor GLIR, largely contributed by GLI3 and the subsequent inhibition of SHH target gene transcription. Many drugs have been developed to control this pathway, which appears deregulated in many neoplasms, particularly for mutations occurring in PTCH and SMO proteins
Almost invariably, BCCs in individuals with Gorlin-Goltz syndrome are caused by a mutation of the PTCH1 gene [43]. In almost all BCCs, a constitutive SHH pathway activity is present, with 90 % exhibiting loss of PTCH1 and 10 % with activating mutations in SMO [44]. However, targeted expression of an activated SMO gene in mice model of BCC demonstrated that the neoplastic precursor was the resident progenitor cell of the interfollicular epidermis and upper infundibulum [5]. By contrast, in irradiated PTCH1+/− mice, BCC arose from stem cells of the follicular bulge [45]. The contrasting results are probably due to additional distinct functions of PTCH1 and SMO, influencing also tumourigenesis outside the SHH pathway. In fact PTCH1 can sequester cyclin B in the cytoplasm [46]. Furthermore, increasing SMO expression seems to be realised also by the loss of p53 functions [47]. Thus, the activation of the SHH pathway either through the loss of PTCH1 or through the expression of a mutated form of SMO is mechanistically distinct. Finally, although the SHH pathway can be activated in cells that do not normally express SMO both through activated SMO overexpression and GLI2 overexpression, the loss of PTCH1 in cells that do not express SMO does not promote similar effects [45]. Finally, also deregulation of G1i2 expression could play a role in the tumour development. In fact increased GLI2 expression in skin stem cells promotes several tumours, depending on the cell of origin and GLI2 expression level [48].
Sporadic BCC
Deregulation of the SHH pathway is also frequently observed in the sporadic BCC. In fact mutations of SMOH or PTCH were reported in most sporadic BCCs, also induced by UVB [49, 50]. The overexpression of Bcl2, main target of SHH pathway, has been widely demonstrated in sporadic BCCs, particularly in indolent-growth subtypes (i.e. superficial and nodular BCCs) [51] (Fig. 5.2). Thus, Bcl-2-induced immortalisation of progenitor epithelia of the hair follicle and the interfollicular epidermis through Bcl-2 predisposes to subsequent mutagenic ‘hits’ from UV light, particularly mutagenesis of p53, mainly observed in aggressive variants of BCC [52] (Fig. 5.3).
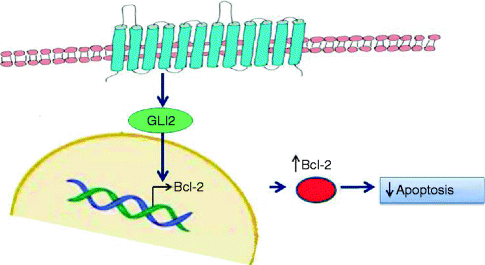
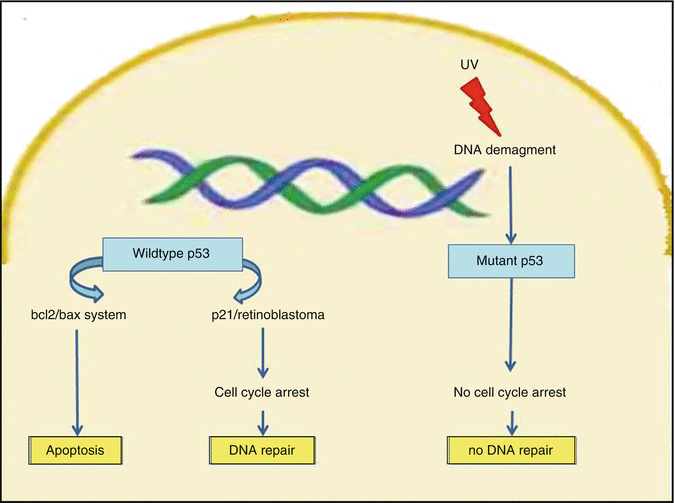

Fig. 5.2
Bcl-2 activation in Hedgehog pathway. Upregulation of Hedgehog pathway, through mutations of involved proteins, results in over-transcription of target genes, particularly Bcl-2, the most efficace antiapoptotic protein

Fig. 5.3
UV light-induced mutant p53. Mutant p53 does not arrest cell cycle, consequently to sublethal genotoxic injury. In consequence, DNA repair does not occur and cells cumulate further genomic instabilities leading to an enhanced malignant potential
In fact p53 mutations have been documented in more than 40 % of aggressive BCCs; occurring in sun-exposed areas, 72 % of the mutations bear the signature of UV light induction [50]. Loss of basement membrane material around tumour cell nests signs the passage from indolent to aggressive BCC growth [52]. The activation of matrix metalloproteinases in this process of transformation guarantees the digestion of basal lamina around tumour nests, promoting the elaboration and/or release of cytokines, also inducing neoplastic cell proliferation [52].
Finally, BCC occurrence after solid organ transplant is significantly higher compared to the remaining population. In addition, in these patients a high frequency of aggressive histotypes and an increased tendency to recurrence and metastasis have been found [53–56]. The pathogenesis of post-transplant BCCs seems to be related to not known virus; in fact herpes virus-like DNA sequences have been demonstrated in some of these cases [53, 54].
Clinical Presentation
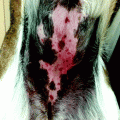
BCCs may have varying sizes from few millimetres to several centimetres. The most common site of occurrence is the head and neck district (Table 5.1). Clinically, five different presentations related to specific histopathological pattern have been described: (1) an opalescent or ‘pearly’ firm nodule, often with adjacent telangiectatic blood vessel, often observed in nodular basal cell carcinoma; (2) a scaly, erythematous, flat lesion with a distinct, sometimes ‘pearl’, border, observed in superficial basal cell carcinoma; (3) a flat lesion, sometimes depressed, white plaque with adjacent erythema in morphoeic basal cell carcinoma (this appearance is similar to lesion of morphoea); (4) depigmented subtype, morphologically similar to the nodular with sclerodermiform features and (5) a pink- or flesh-coloured nodule with a constricted inferior margin suggesting a seborrhoeic keratosis in fibroepithelioma, a rare form of BCC preferably located in the lumbosacral, pubic of genitocrural region [57–59].
The tumoural growth is generally slow, but rapid enlargement with ulceration and bleeding could occur. Local infiltration of perilesional extracutaneous tissues is rarely observed, dependent on tumoural anatomic sites, but metastasis is rarely recorded. Clinical differential diagnosis with other cutaneous tumours arises and only histological examination categorises the lesions correctly. For example, extensive pigmentation in BCC can be interpreted as a malignant melanoma.
Histopathology
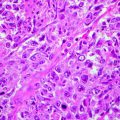
Several histological subtypes of BCC are distinguished, and such a variety is related to the fact that basal cells can differentiate in both epidermis and adnexal structures.
BCC can be divided into two groups: undifferentiated and differentiated. The differentiated BCCs show a slight degree of differentiation toward the cutaneous appendage. However, a clear cut between the two groups cannot be made, because many undifferentiated basal cell carcinomas show focal differentiation in some areas and most differentiated basal cell carcinomas show areas lacking differentiation.
Some specific features are quite common to all BCC subtypes. Thus, six histological features are generally found in all BCCs: (1) nests of basaloid cell, resembling the epidermal basal row keratinocytes; (2) peripheral palisading of the nests, an expression of residual polarity of the tumoural basal cell; (3) high mitotic rate; (4) individual necrotic keratinocytes; (5) an unusual cellular stroma composed of spindle cell in a mucinous matrix with fine collagen fibrils and many mast cells; and (6) cleft formation, separating tumoural nests from their stroma, attributable to collagenase activity, mucin deposition and a reduction in the number of hemidesmosomes [60]. Some of these features can be attenuated in specific variants.
Additional common features are keratinisation and pigmentation. Pigmentation attributable to the proliferation of dendritic, heavily pigmented melanocytes along with the keratinocytes and the transfer of pigment also to neoplastic keratinocytes is mainly observed in superficial basal cell carcinoma [61].
The stroma is a determinant for BCC development, as it survives only when a broad representation of the stroma is present; for this reason the palms of the hands and soles of the feet will most likely not contain the elements necessary for the development of neoplasia. Stroma closed to tumoural nests contains many young fibroblasts, and areas of retraction of the stroma from the tumour island result in peritumoural lacunae.
BCC can be also found in clinical syndromes. The multiple basal cell carcinomas seen in the nevoid basal cell carcinoma syndrome (Gorlin-Goltz syndrome) present no distinctive features compared to ordinary basal cell carcinoma, even while they are still in the early nevoid stage and have not yet become invasive and destructive [62]. The BCCs encountered in the Bazex syndrome have a variable histological appearance. Some of them are indistinguishable from trichoepithelioma [52, 63]. Table 5.2 reports the frequency of the most common subtypes in two wide European series.
Undifferentiated BCCs
Among undifferentiated BCCs, two clinical groups can be considered, i.e. indolent-growth variants, as superficial BCC and nodular BCC, and aggressive variants, as infiltrative BCC, metatypical BCC and morpheiform or sclerosing BCC [52] (Figs. 5.4 and 5.5).
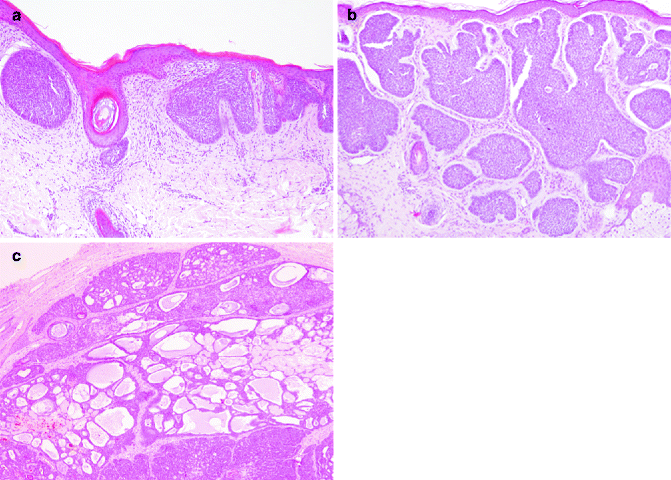

Fig. 5.4




Undifferentiated indolent BCC subtypes. (a) Superficial BBC is characterised by nests connected to epidermal surface (H&E 10×); (b) nodular BCC is characterised by nodular nests sparsed in the dermis (H&E 10×); (c) adenoid-cystic areas could be observed in nodular BCC (H&E 10×)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree