Fig. 33.1
Frontal (a) and lateral (b) view in a dog with diffuse and infiltrative SCC of the nasal planum. Please note marked depigmentation and asymmetry associated with the lesions
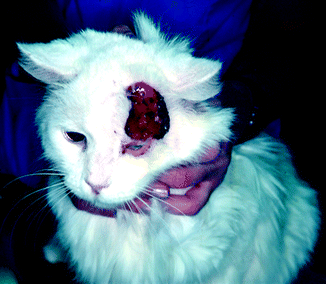
Fig. 33.2
White-coated cat with advanced, aggressive cutaneous SCC lesion and progressing to the periorbital area
Biologic Behavior
Canine SCC of the oral cavity has site-associated metastatic behavior, with tumors of the rostral aspect of the mouth less prone to metastasis than those of the caudal tongue, oropharynx, and tonsil [49]. However, this site-associated metastatic behavior has not been reported for cSCC. Prognostic factors for cSCC in veterinary medicine related to recurrence and metastasis are unknown. In humans, the most important factors affecting risk of recurrence and metastasis are the size and location of the primary tumor. Large lesions, considered to be those greater than 2 cm in diameter, recur at a rate of 15 %, which is twice that of smaller lesions. These larger lesions also metastasize at a rate of 30 %, three times that of smaller lesions [50]. Squamous cell carcinomas of the human lip and ear are also aggressive lesions, with rates of recurrence and metastasis ranging from 10 to 25 % [50, 51]. Other sites associated with a high risk of recurrence and metastasis in humans are eyelid, nose, and mucous membranes [52, 53]. Locally recurrent squamous cell carcinomas metastasize at rates that range from 25 % for most cutaneous lesions to 30–45 % for ear and lip tumors [50, 52]. Squamous cell carcinomas arising in injured, chronically diseased or chronically inflamed skin can also demonstrate more aggressive clinical behavior and a greater propensity to metastasize, with an overall metastatic rate of 40 % [50, 54]. Clinical features associated with recurrence and metastases include rapid growth and local recurrence of the tumor as well as immunosuppression [52]. Histologic features that are predictive of recurrence or metastasis include a depth of more than 4 mm, involvement of the reticular dermis or underlying tissues. Poorly differentiated cSCC in humans recurred at a rate of 28.6 %; in contrast, well-differentiated tumors had a local recurrence rate of 13.6 % [50].
However, despite the fact that the majority of these tumors present at early stages, cSCC accounts for the majority of non-melanotic skin cancer deaths and 20 % of all skin-cancer-related deaths in humans [50, 55]. For those with metastatic disease, however, the long-term prognosis is extremely poor [56]. In humans, if metastasis does occur, regional lymph nodes are involved in approximately 85 % of cases; approximately 15 % of cases involve distant sites, including the lungs, liver, brain, skin, and bone [56, 57].
Most veterinary patients with primary sunlight-induced cSCC have a good-to- excellent prognosis when lesions are detected and addressed early in the course of disease. Lesions occurring on sun-exposed skin have better prognosis than those occurring in unexposed skin. In North America, dogs and cats affected by sunlight-induced cSCC are often kept outdoors, and lesions may not be detected in early stages. Also, owners may be less willing or financially unable to seek veterinary care for their outdoor pets, when the lesions are most manageable. Fortunately, cSCC lesions in dogs and cats are slow to metastasize. Small and superficial lesions may never progress, whereas more deeply invasive lesions can become metastatic by the lymphatic route primarily. Ultimate visceral metastasis can be seen in end-stage cases [47].
Diagnosis
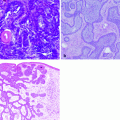
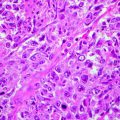
Cytology may allow differentiation of neoplastic from inflammatory lesions and of epithelial from spindle cell tumors. Cytologic diagnosis of ulcerative lesions may be problematic, as inflammation can induce secondary proliferative changes in epithelial cells even in benign lesions. Moreover, ulcerative lesions may be associated with lymphadenopathy, which may be reactive or may represent metastasis to regional nodes in cases with cSCC. Cytologic examination of enlarged regional lymph nodes should be performed before definitive therapy. Histologic examination of the tumor is important for further treatment planning and identifies possible prognostic factors associated with the suspected malignancy such as grade or level of cellular differentiation. In addition, histopathology allows determination of invasion and whether surgical excision was adequate. Cutaneous lesions are rarely metastatic to visceral organs; however, systemic staging for pulmonary metastasis is indicated for patients with advanced local disease or nodal metastasis. Advance local staging with CT scanning may be helpful for surgical and radiation therapy planning [47].
Treatment
Cutaneous SCC lesions may be locally aggressive but are slow to metastasize. Thus, adequate local control may be curative for these tumors, particularly when they are addressed early in the clinical course of disease. Local modalities of treatment include complete surgical excision as the most cost-effective and successful means of local control. Complete excision with 1–3 cm surgical margins may be curative for most lesions. This might necessitate ear pinna amputation in cats, or nasal planum resection, which owners may consider unacceptably disfiguring. For lesions not amenable to resection due to size or location, radiation therapy, cryosurgery, electrochemotherapy, photodynamic therapy, or intralesional chemotherapy may be helpful.
Cryosurgery
Cryosurgery is a minimally invasive procedure that destroys malignant tissue through inducing cell death by formation of intracellular and extracellular ice crystals. Liquid nitrogen, argon, and dimethyl ether-propane are frequently used as cryotherapy agents. The agent is directly applied to the skin or introduced by cryoneedles for delivery. Most canine and feline cSCC treated with cryosurgery achieve good-to-excellent overall remission rates (80 %), although many required multiple treatments and development of recurrence is observed in up to 73 % of cases. Recurrence of the malignancy is mostly associated with degree of infiltration (deep vs. superficial) and volume size (>0.5 cm in diameter) of the mass [48, 58, 59].
Most of the adverse effects associated with this low-cost therapy, including erythema, bleeding, blisters, and minimal pain, are localized and well tolerated by the patient.
Electrochemotherapy
Electrochemotherapy is a form of localized treatment for tumors that involves administration of a chemotherapeutic agent combined with delivery of appropriate energy waveforms. The ultimate goal of those waveforms is to induce an increased uptake of the drug by cancer cells. One published manuscript for treatment of cSCC describes the local administration of bleomycin (1–1.5 mg/cm3 of lesion) followed in 5 min by permeabilizing biphasic electric pulses. Eight pulses of 50 + 50 μ (microns) at 1,300 V/cm3 are delivered by a pair of electrodes. Total treatment consists of two sessions of electrochemotherapy 1 week apart. Toxicity reported was described as mild erythema localized to tumor lesion and was transient and well tolerated by cats. Almost 80 % (7/9 cases) of the patients had a complete response (CR), and 55 % (5/9) had durable control lasting more than 1 year [60, 61].
Intralesional Chemotherapy
This treatment modality involves the direct intratumoral delivery of chemotherapeutic agents. Site-directed administration of the drug is intended to achieve local control of malignancy by providing a higher tumor-to-plasma ratio of the drug over a prolonged period of time. An additional advantage of this therapy is reduced to absent systemic adverse effects.
Cisplatin (1 mg/cm3 of tissue target in oily emulsion) and bleomycin (1 mg/cm3 of tissue) four times a week on a 2-week interval has proven efficacious in treatment of cSCC in horses. The local control rate at 1 year for lesions treated with cisplatin was approximately 93 % and with bleomycin was approximately 78 % [62, 63]. Cisplatin in cats at standard IV doses (50–70 mg/m2) is lethal through induction of severe pulmonary edema. However, local injection as repositol implants, consisting of purified cosmetic-grade bovine collagen matrix (20 mg/ml), epinephrine (0.1 mg/ml), and the chemotherapy agent (cisplatin 3.3 mg/ml), resulted in 86 % CR and 4 % partial response (PR) after 2 weekly treatments on average for a study population of 17 cats with a total of 51 cSCC lesions. Average disease-free interval (DFI) was 10.5 months with a local recurrence rate of 30 % in these cats (B. Kitchell 1994, personal communication).
The antimetabolite chemotherapeutic agent fluorouracil (5-FU) inhibits both RNA and DNA synthesis and targets dividing cells. Fluorouracil in cats at standard IV human doses (400–600 mg/m2) induces rapidly lethal neurotoxicity. In fact, it is possible to see fatal neurotoxicosis in cats with the use of 5 % topical fluorouracil cream to the ear tips [64].
One study described the use of collagen implants as described above, substituting fluorouracil (5-FU 30 mg/ml) for cisplatin in the formulation. Using a regimen of 3 weekly implants, 58 % CR and 17 % PR were noted for a study population of six cats with a total of 16 lesions. Average DFI was 5 months with local recurrence rate of 14 %. Mild-to-moderate local adverse effects such as erythema and desquamation were noticed (B. Kitchell 1994, personal communication).
Intralesional delivery of chemotherapy in purified bovine collagen with fluorouracil as described above was applied to 13 dogs with sunlight-induced cSCC with 100 % overall response rate (54 % CR, 46 % PR). Cisplatin (3.3 mg/ml) in the collagen gel implant was given sequentially when CR was not achieved with fluorouracil, and no further reduction in area of the tumor was apparent after 2 weekly injections of fluorouracil implants. Cisplatin implants achieved CR that provided DFI of 44 months in two cases that achieved initial PR after treatment with fluorouracil implants. Partial remission allowed complete surgical excision to achieve cure in 3/6 dogs. The average DFI was almost 50 months. Further, weekly fluorouracil or cisplatin collagen implant treatments were well tolerated with minimal local erythema and no systemic adverse effects [65, 66].
Photodynamic Therapy (PDT)
This treatment modality consists of intravenous injection of an inert photosensitizer that is activated by light at the appropriate wavelength (600–900 nm “therapeutic windows”). Upon activation, the photosensitizer can undergo type I reaction, reacting directly with substrates such as DNA. Alternately, the photosensitizer can undergo type II reaction, directly producing free radicals or interacting with molecular oxygen to generate cytotoxic reactive oxygen species.
In a previous study, six feline cSCC were treated, resulting in two partial responses and four long-term complete responses with DFI that ranged from 276 to 576 days. Toxicity was described as tolerable and mostly localized to the skin. Erythema was noted and ulceration was occasionally observed, especially when PDT was applied for lesions of the eyelid. Systemic toxicity included nausea associated with the photosensitizer injection and elevated body temperatures 2 days after PDT. In one cat, anorexia and peripheral neuropathy of undetermined cause noted 2 weeks after PDT resolved without treatment [67]. Another study described the use of a novel liposomal photosensitizer for PDT in 18 cats. Local toxicity consisting of erythema and edema was reported in 15 % of the patients, and the CR rate was100 %. The overall 1-year control rate was 75 %. The tumor recurrence rate in this cohort of patients was 20 % with a median time to recurrence of 172 days [68]. Similar response rates (overall response rate 96 %; CR 84 % and PR 11 %) to a single treatment with topical photosensitizer (5-ALA) were observed by Bexfield et al. [69] in a population of 56 cats with nasal planum cSCC. Recurrence was noticed in 51 % of the cases, with a median time to recurrence of 157 days. At a median follow-up of 1,146 days, 45 % of cats were alive and disease-free but 33 % had to be euthanized due to local tumor recurrence. In this study, erythema and edema were observed in all cats after treatment, but these adverse effects were localized, mild, and transient. The lesions appeared to cause some discomfort, as manifested by occasional rubbing of the nasal planum [69].
Topical Therapy
Imiquimod is an antiproliferative agent and immune system stimulator. Imiquimod acts as a Toll-like receptor 7 agonist. Activation of this receptor protein plays a fundamental role in pathogen recognition and activation of innate immunity. Imiquimod is available as a topical cream and has been used to treat in situ cSCC presented as multiple lesions not invading the basal layer of the skin [70]. Imiquimod 5 %, used once daily on an alternate-day dosing regimen was associated with 100 % (40 % CR, 60 % PR) response rate in 12 cats affected by in ISSSC. Because of multifocal nature of the disease, appearance of new masses was observed in 75 % of the cats included in the study, and treatment duration extended to approximately 300 days on average. New lesions also responded to treatment in all cats. Toxicity was reported in 40 % of the cats. The toxicity observed included local erythema, mildly increased liver enzymes, mild neutropenia, anorexia, and vomiting [71–73].
Radiation Therapy
Most of the published data is focused on radiation therapy for cSCC of the nasal planum in cats. The volume of cSCC lesion treated was inversely associated with DFI and survival time [74]. Orthovoltage fractionated radiotherapy given as a total dose of 40 Gy provided a 1-year progression-free survival rate of 60 % in 90 cats with nasal planum SCC [74]. Proton therapy achieved similar control rates to orthovoltage radiotherapy with an overall response rate of 93 % in 15 cats with nasal planum SCC [75]. Plesiotherapy is a direct application of a strontium-90 radiation source to superficial cutaneous lesions. In this therapy, 50 Gy of radiation is delivered to a depth of 2 mm and administered in five fractions over a 10-day period to small (2–5 cm diameter) superficial lesions. This treatment achieved 87 % complete remission with no local recurrence noted for 2 years in 15 cats with cSCC [76]. Radiotherapy was not as effective in controlling residual cSCC in seven dogs that failed surgical curative-intent resection. Radiation therapy alone in three dogs only provided control of the disease for no longer than 8 weeks as an average [77].
The retinoic acid drugs called retinoids are derivatives of vitamin A, which is an essential factor for epithelial cell differentiation. Retinoids have been demonstrated to induce growth inhibition of premalignant lesions such as actinic keratoses, by induction of terminal differentiation, apoptosis, and cell cycle arrest [78]. Administration of etretinate to ten dogs at 1 mg/kg twice daily for a minimum of 90 days induced complete resolution of preneoplastic lesions in two dogs and partial responses in three dogs. Treatment toxicity included reversible hypertriglyceridemia and transient serum liver enzyme elevations in three dogs [79].
Systemic chemotherapy is not typically used to treat cSCC, as these lesions are rarely systemically disseminated. Agents such as carboplatin, bleomycin, fluorouracil, and doxorubicin have been administered with limited effect. The use of nonsteroidal anti-inflammatory agents such as piroxicam has been used in adjunctive protocols, as there is some evidence of tumor regression in oral SCC in the dog. Pain control afforded by NSAIDS may have a palliative effect for the patients, even if direct anticancer effect is not noted [80].
Future Treatment Strategies
The signal transducing G-coupled peptide Hras upregulates Fyn mRNA, and this suggests the potential for an interesting biologic relationship between Ras and Fyn in cutaneous neoplasms such as cSCC. Ratushny et al. [4] proposed the topical application of small-molecule kinase inhibitors (SMKIs) that have the physical properties required to penetrate the skin. The ideal SMKIs would target Fyn and related tyrosine kinases or would target kinases in the Ras pathway. The tyrosine kinase inhibitor dasatinib is smaller than 500 Da in molecular size and targets multiple tyrosine kinase receptors, including Fyn. Topical dasatinib has been proposed as a possible therapeutic agent in this context [81]. Metronomic chemotherapy protocols involving the use of the oral receptor tyrosine kinase inhibitors toceranib and masitinib have some modest evidence of efficacy against oral SCC lesions, which might prove helpful in cSCC management as well [82].
Cutaneous Hemangiosarcoma
Incidence and Risk Factors
Hemangiosarcoma is a malignancy of vascular- or vessel-forming cells. The median of age range of dogs at the time of diagnosis of hemangiosarcomas affecting the cutis (cHSA) is approximately 10 years; in cats the median age at diagnosis is approximately 12 years [20, 83–85]. There is no known sex predilection, but most cutaneous vascular tumors (hemangiomas and hemangiosarcomas) in cats have been reported in males [20, 45, 83–85]. Hemangiosarcomas of the skin have predilection for cutaneous over subcutaneous tissue and for glabrous, lightly pigmented skin when compared with haired skin. Dogs with short hair coats and lightly pigmented skin have more hemangiomas and hemangiosarcomas of the cutis than do dogs with variable length hair coats and pigmentation [20]. Cutaneous HSA is commonly observed in predisposed breeds such as American Staffordshire terriers, pit bulls, beagles, Dalmatians, Italian greyhounds, whippets, and bull terriers [20, 83, 85]. Outdoor cats with unpigmented skin may be predisposed to cutaneous tumor development in areas without adequate pelage, particularly on the pinna and head [84].
Cutaneous HSA usually presents as solitary or multiple small cutaneous lesions, often less than 1 cm in diameter. In a recent report of 94 dogs with suspected or confirmed diagnosis of cHSA, 71 % of the cases at initial diagnosis had one solitary dermal lesion, and 29 % had multiple cutaneous lesions [20, 83]. Whether the presence of multiple sites of cHSA is a result of metastasis or whether the lesions arise de novo as multiple primary tumors is unclear.
Biologic Behavior
Recently, Szivek et al. [83] were able to identify prognostic factors that predict outcome for cHSA in dogs. Identified prognostic factors included breed, tumor location, and the presence of solar actinic changes. Predisposed breeds were found to have a median survival of 1,570 days compared with 593 days in non-predisposed or atypical breeds. Predisposed breeds had a lower metastatic relative risk of 0.45 when compared with non-predisposed breeds. Tumor location predicted survival of dogs with cHSA. Dogs with lesions arising in typical sun-exposed ventral abdominal locations had a median survival of 1,085 days compared with 539 days for dogs with tumors seen in other body locations. Tumor location also predicted locoregional recurrence. The solar-induced form associated with actinic changes has a reported median survival of 1,549 days compared with 545 days in dogs without actinic lesions [83]. The prognostic significance of association of solar elastosis or actinic changes with outcome of cHSA is controversial in the literature, however [83, 85]. Dogs of non-predisposed breeds with tumors in areas other than the ventrum, and that also lack actinic changes, or those with subcutaneous involvement appear to have a more aggressive form of HSA with higher risk of developing visceral HSA. Dogs with subcutaneous invasion have an associated metastatic relative risk of 2.04 when compared to dogs with only cutaneous involvement [83].
Lesions confined to the dermis are correlated with better prognostic outcome and lower recurrence rates, possibly because of ease of surgical excision [85]. Cutaneous HSA lesions may be easier to excise completely, and recurrence is much less frequent than for tumors involving deeper tissues. Locoregional recurrence is very common in predisposed thin-coated breeds with the solar-induced form of this disease. The incidence of metastasis is considered to be low in cHSA in dogs and cats, but some patients may ultimately develop metastatic disease. Metastasis was documented or suspected in 34 % of dogs with cHSA and occurred at a median of 326 days from diagnosis. Progression to the visceral form of HSA was observed in 62 % of dogs with metastatic disease. These dogs are frequently diagnosed by the presence of hemoabdomen that occurs in more than the 95 % of the cases with visceral metastasis. Overall, the median survival for dogs treated by surgical excision with no adjuvant chemotherapy was reported to range from 780 to 987 days, with 1-, 2-, and 3-year survival rates of 79, 60, and 44 %, respectively. Median survival was 1,095 days in surgically treated cats with cHSA [20, 83, 84].
Diagnosis
Cutaneous HSA lesions closely resemble benign hemangiomas of the dermis, in both gross and cytologic appearance. Therefore, a cytologic approach may not be adequate for accurate diagnosis. Histologic examination allows determination of invasion and whether surgical excision was adequate. Systemic staging for pulmonary and visceral metastasis is indicated based on potential for spread to distant organs. Advanced local staging with computed tomography scanning may be helpful for surgical and radiation therapy planning.
Treatment
Surgery
Cutaneous HSA lesions are most often treated with curative-intent surgical excision. When patients have cHSA present in multiple sites, multiple surgeries are required. Locoregional recurrence was documented in 77 % of the cases at a median time of 211 days after initial diagnosis. Locoregional recurrence only occurred in skin anatomically close to the previously resected tumor. Predisposed breeds were found to be more affected by the development of locoregional recurrence, particularly when masses were located on the ventrum, and also for dogs with multiple masses at initial presentation (Fig. 33.3). Biopsy margin status documenting complete surgical excision, absence of metastasis, and no subcutaneous invasion were surprisingly unassociated with incidence of local recurrence [83]. This may represent the effect of solar field cancerization, in which case all cells in the solar-exposed field have increased risk of carcinogenesis [86]. The dearth of prospective studies defining the clinical behavior of cHSA results in uncertainty and controversy regarding the role of complete surgical excision of the lesion as a predictor of clinical outcome in dogs and cats [83, 85].
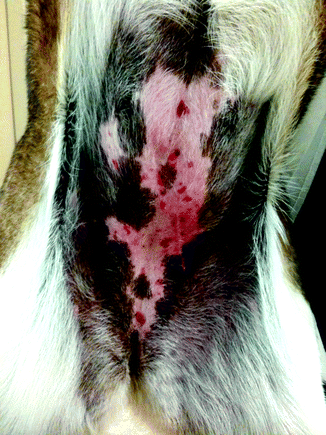

Fig. 33.3
Multiple small cutaneous HSA lesions located in the unpigmented skin of the ventral abdomen of a dog
Chemotherapy
Use of chemotherapy in the management of dogs and cats affected by cHSA is largely recommended only if there is an evidence of invasion of the subcutaneous tissue or of distant metastasis. Recent studies have documented a possibly higher metastatic potential than previously believed in cats with cHSA [20, 83, 87]. Doxorubicin-based protocols provide several more months of survival time for these cats, when compared to no therapy. The most common chemotherapeutic agent used to treat systemic HSA is doxorubicin. Doxorubicin may be administered as a single agent every 2 or 3 weeks at 30 mg/m2 for dogs, and 20–25 mg/m2 or 1 mg/kg for dog <10 kg or for cats affected by cHSA with poor prognostic features. Doxorubicin may be used as described above in combination with cyclophosphamide 200–250 mg/m2 IV, or 50 mg/m2 PO for 4 days during week 1, and vincristine 0.5–0.7 mg/m2 on days 8 and 15 of a 21-day cycle (VAC protocol). The DAV protocol substitutes the alkylating agent dacarbazine (DTIC) for cyclophosphamide in the VAC protocol. In the DAV protocol, dacarbazine is delivered at 800 mg/m2 as an 8-h infusion diluted in 0.9 % NaCl at maintenance rate on day 1, with doxorubicin and vincristine delivered as scheduled for the VAC protocol. The DAV protocol has been used for treatment of visceral HSA. However, no chemotherapy protocol has been proven superior when compared to single-agent doxorubicin. More intense protocols such as VAC and DAV are associated with more toxicity [88–90].
Other therapies used to treat visceral HSA include ifosfamide delivered at a dose of 350–375 mg/m2 IV every 3 weeks as a single agent. Complete response with tolerable toxicity was observed in a dog with metastatic cHSA [91].
Targeting tumor-associated neovasculature is the object of many anticancer therapeutics strategies. These investigations have been conducted in dogs with HSA and include studies of the efficacy of doxorubicin nanoconjugates that target transmembrane proteins expressed in the neovasculature of cHSA and other solid tumors. In vitro and in vivo studies using nanoconjugates showed measurable anticancer activity in cHSA cell lines and in xenograft mouse models implanted with canine cancer cells. These promising preliminary results warrant further investigation on macroscopic solid tumors [92].
Radiation Therapy
The use of radiation therapy for treatment of cHSA has not been extensively explored in dogs and cats. Palliative radiation therapy has been reported to have relative success in controlling nonsurgical cutaneous bleeding masses. The treatment protocol consisted of 3–4 Gy fractions to achieve a 24 Gy total radiation dose [90]. Other investigators have suggested the use of radiation therapy for control of cutaneous cHSAs with incomplete surgical resection. These studies reported poor responses in two cats with large, unresectable cHSA lesions [93].
Future Treatment Strategies
A newer concept in chemotherapy drug delivery is referred to as metronomic chemotherapy. Metronomic therapy is based on continuous drug exposure to susceptible cancer cells that results in direct inhibition of tumor cell replication, as well as inhibition of angiogenesis and alteration of immune function. The metronomic strategy is attractive as a cost-effective and well-tolerated treatment alternative for veterinary patients with malignancy.
Most of the metronomic treatment protocols in common veterinary use consist of combinations of NSAIDS and oral alkylating agents. Oral alkylating agents such as cyclophosphamide at 15–25 mg/m2 or chlorambucil at 4 mg/m2 may be given daily or on alternate days in combination with the oral administration of an NSAID such as piroxicam at the dose of 0.3 mg/kg daily. Increased benefit also appears to occur when metronomic chemotherapy is used in combination tyrosine kinase inhibitor drugs, such as toceranib and masitinib [94].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree