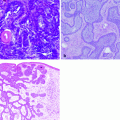
Fig. 1.1
Histological section of human skin. The human skin is composed by a superficial upper layer, the epidermis covering a deeper layer, the dermis. Inside the dermis, numerous glands (arrows), blood vessels (bv), and hair follicles (hf) are evident. Below the dermis is visible the subcutaneous fat (stars). Mallory trichromic stain. Original magnification 2.5×
The two skin layers are interconnected with each other through epidermal-dermal junctions. These are undulating in section and formed by ridges of the epidermis, known as rete ridges, that project into the dermis. The junction provides mechanical support for the epidermis and acts as a partial barrier against exchange of cells and large molecules. Below the dermis, there is a fatty layer, the panniculus adiposus, usually designated as “subcutaneous.” This is separated from the rest of the body by a vestigial layer of striated muscle, the panniculus carnosus [4].
There are two main kinds of human skin. Glabrous skin (non-hairy skin), covering the palms and soles, is grooved on its surface by continuously alternating ridges and sulci. It is characterized by a thick epidermis divided into several layers, including a compact stratum corneum; by the presence of encapsulated sense organs within the dermis; and by a lack of hair follicles and sebaceous glands. On the contrary, hair-bearing skin has both hair follicles and sebaceous glands but lacks encapsulated sense organs [4].
The integumentary system has not only principally protection functions but also thermoregulation, respiration, and perception functions [1]. The skin is the first line of defense against environmental (mechanical, chemical, osmotic, thermal) insults and microbial infection, as well as water and electrolyte loss [2, 3]. It confronts these attacks by undergoing continual self-renewal to repair damaged tissue and replace old cells [5]. Stem cells are located in the adult hair follicle, sebaceous glands, and in the basal layer of the interfollicular epidermis [5, 6]; they have the function to maintain tissue homeostasis, regenerating hair and repairing the epidermis after injury [5].
Embryology of the Skin
The skin develops by the juxtaposition of two embryological elements: the prospective epidermis, which originates from a surface area of the early gastrula, and the prospective mesoderm, which is brought into contact with the inner surface of the epidermis during gastrulation [4, 7, 8].
The epidermis originates almost completely from the covering ectoderm, and only few cells (melanocytes and Langerhans’ cells) migrate from other areas. On the contrary, the dermis develops from two different mesenchymal areas; the larger part arises from somatopleure (lateral mesoderm) and the smaller part arises from somites (paraxial mesoderm) [1]. Both components of the skin should be considered as donors and receptors of information. Morphogenesis of the skin depends on a careful and constant dialogue between them [9]. Before skin morphogenesis, several cell interactions take place, in order to specify first the formation of dermal progenitors [10, 11] and second their densification inside the sub-ectodermal space [9]. These two first steps lead to the formation of the embryonic skin, formed by an upper epidermis overlying a dense dermis. The next step is the initiation and organization of regular repetitive appendage primordia. Finally, the final step is the organogenesis of the epidermal primordia ( placode) in a complete, mature appendage [9].
Development of the Epidermis
After gastrulation, the embryo surface emerges as a single layer of neuroectoderm, which will ultimately specify the nervous system and the skin epithelium [5]. The covering ectoderm develops from epiblast during the third week of fetal life and represents the ectoderm that does not differentiate in nervous tissue. During the fourth week, the covering ectoderm separates from neural tube and closes on nervous system, forming a continuous coating on embryo surface. At the crossroads of this decision is Wnt signaling, which blocks the ability of ectoderm to respond to fibroblast growth factors (FGFs) [5]. In the absence of FGF signaling, the cells express bone morphogenetic proteins (BMPs) and become fated to develop into epidermis [5] (Fig. 1.2). Initially, the covering ectoderm is composed by a single layer of undifferentiated, cuboidal, and glycogen-filled cells [4, 12] (Fig. 1.3a). At this early stage, cell proliferation is the dominant process [13]. At the end of the fourth week, the epidermis forms a second layer that lies outer and originates simple squamous epithelium called periderm, a purely embryonic structure, which is unique to primates [4] (Fig. 1.3b). Its cells flatten, cornify, and eventually spread to several times the diameter of the deeper cells. The inner, basal layer includes stem cells and represents the germinal layer (stratum germinativum) of epidermis, whereas the periderm establishes the protection barrier on contact with amniotic fluid [1].
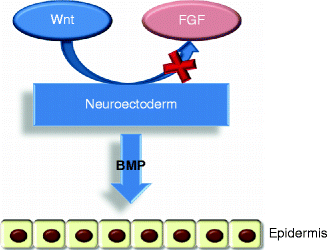
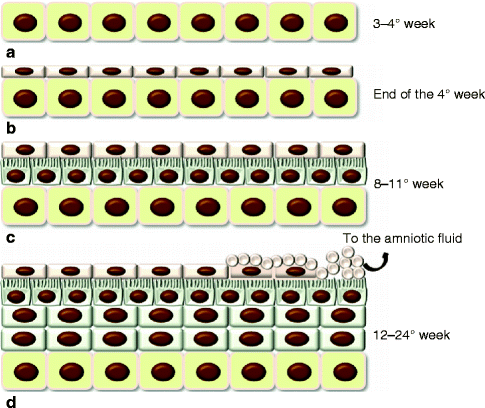

Fig. 1.2
The epidermis formation. Wnt signaling blocks FGF activity on the neuroectoderm that can express BMP proteins so developing the epidermis

Fig. 1.3
Development of the epidermis. (a) The covering ectoderm is formed by a single layer of cuboidal, undifferentiated cells; (b) the epidermis is composed by a second superficial layer called periderm; (c) the germinal basal layer originates a third middle layer, the intermediate layer, whose cells are characterized by the presence of microvillus projections at the surface of the periderm; (d) during the fifth month, the intermediate layer proliferates, forming one or more other layers. The periderm cells form numerous blebs and get away in the amniotic fluid (See more details in the text)
Between 8 and 11 weeks, the germinal layer actively proliferates and originates a third middle layer, the intermediate layer (Fig. 1.3c). Development of this layer is associated with asymmetric cell division of embryonic basal keratinocytes [14–16]. Glycogen is abundant in all layers, and a few microvillous projections occur at the surface of the periderm. The surface cells are flat and polygonal [4, 17]. These three layers persist about a month and then the epidermis later on evolves. During the fifth month (12–16 weeks), there are one or more intermediate layers (Fig. 1.3d). These cells contain mitochondria, Golgi complexes, and a few tonofilaments, as well as abundant glycogen both within and between cells. Microvilli become much more numerous [4]. From this stage onward, dome-shaped blebs start to project from the centers of the periderm cells. At first, the blebs are simple, but later their surface becomes dimpled and infolded [4]. The periderm gets away, and in some weeks (by 24 weeks), it is removed in the amniotic fluid, forming, together with shed lanugo, sebum, and other materials, the vernix caseosa [4]. The periderm may be no more than a protective coating for the fetus before keratinization of the epidermis. On the other hand, features such as the abundant microvilli, raised blebs, coated- and smooth-membrane vesicles, and increasing cell size suggest that it may have an important function such as the uptake of carbohydrate from the amniotic fluid [4, 17].
In the same time, like basal keratinocytes, intermediate cells undergo proliferation and/or differentiation. The loss of their proliferative capacity is associated with the maturation of intermediate cells into spinous cells [14, 16, 18]. The basal layer together with the spinous layer forms the Malpighian layer [19]. The spinous layer cells undergo further maturation to form the granular layer (stratum granulosum) and the outer cornified layer (stratum corneum) [1, 18].
In the epidermis of the hand and foot, among granular and cornified layers, a thin additional layer, called bright (glossy) layer (stratum lucidum) for its refraction property, lies. This layer is formed by cells containing the fluid eleidin that replaces the granules. All the epidermis layers are formed by cells called keratinocytes because they contain keratin by 14 weeks [4]. The germinal layer continuously produces cells that differentiate in keratinocytes, move toward the upper layers, degenerate, and finally are eliminated in the environment. During this migration among layers, keratinocytes pass through several maturation phases and their structural transformations originate morphological differences. Hemidesmosomal and desmosomal proteins are already detectable in the basal keratinocytes at 10 weeks [4].
Epidermal cells must undergo growth arrest before they can initiate a differentiation program [13]. Moreover, the morphological changes that are a hallmark of epidermal stratification are associated with changes in the expression of keratin differentiation markers [16, 20]. In fact, during normal epidermal development, commitment to the epidermal lineage involves the repression of the non-epidermal keratin pair K8/K18 [16, 21] and the induction of the epidermal keratin pair K5/K14 [6, 16, 22, 23]. Keratinocytes belonging to the spinous layer are big and polyhedric cells, synthesizing high quantity of keratin and keratohyalin, the two specific proteins of the epidermis. In the granular layer, these proteins are organized in two several types of subcellular aggregates: keratohyalin granules and keratin bundles. Some derivatives of keratohyalins, particularly the filaggrin, used to tightly join cells together, are first detectable at 15 weeks [4]. Moreover, cells forming the layer just beneath the periderm become to express loricrin [13, 23–25]. Specifically, keratohyalin granules appear at 21 weeks in the uppermost layer [4]. The initiation of terminal differentiation results in the induction of K1 and K10 expression in the newly formed suprabasal keratinocytes [18, 26, 27]. In addition, cornified envelope proteins, which are rich in glutamine and lysine residues, are synthesized and deposited under the plasma membrane of the granular cells [5]. When the cells become permeabilized to calcium, they activate transglutaminase, generating γ-glutamyl-ε-lysine cross-links to create an indestructible proteinaceous sac to hold the keratin macrofibrils (including various keratins, involucrin, loricrin, and filaggrin) [5, 13]. In the higher part of granular layer, the cells start to show the first signs of terminal differentiation and degeneration: flattening cell form, destruction of cellular organelles, dense chromatin granules, and breaking of the nuclear envelope. When the cells pass into the cornified layer, they completely degenerate, lose nuclei, and assume the shape of flattening sacs full of keratin, forming 15–30 layers of dead cells. Terminal differentiation is a slow process that requires many newly synthesized proteins in all layers of the epidermis [13].
The plane of union between epidermis and dermis is smooth until early in the fourth month when epidermal thickenings grow down into the dermis of the palm and sole. About 2 months later, corresponding elevations first appear on the skin surface. These epidermal ridges complete their permanent, individual patterns in the second half of fetal life [28].
The Regulation of Epidermal Development
The process during which the unspecified surface ectoderm adopts an epidermal fate is defined as epidermal specification [16].
Generally, keratinocytes take about 4 weeks to pass from the germinal layer to the outside of the body, and the epidermis structure depends on both their proliferation rate and differentiation processes. The fine balance among proliferation and differentiation is regulated by a complex system of interaction between many growth factors (Table 1.1). Some of these stimulate keratinocyte proliferation (epidermal growth factor, fibroblast growth factor, transforming growth factor-α, insulin, and interleukins), whereas others inhibit it (transforming growth factor-β1 and transforming growth factor-β2, interferons, and tumor growth factor) [1]. These pathways may be variably activated, both spatially and temporally, leading to a diverse series of transcribed genes [4].
Table 1.1
Main growth factors involved in proliferation and differentiation of keratinocytes
Proliferative | Anti-proliferative |
|---|---|
EGF | TGFβ1 |
FGF | TGFβ2 |
TGF-α | Interferons |
Insulin | Tumor growth factor |
Interleukins | NF-κB |
KGF |
TGF-α is made by the basal cells and stimulates their own division. If the TGF-α gene is linked to a promoter for keratin 14 (one of the major skin proteins expressed in the basal cells) and inserted into the mouse pronucleus, the resulting transgenic mice activate the TGF-α gene in their skin cells and cannot downregulate it. The result is a mouse with scaly skin, stunted hair growth, and an enormous surplus of keratinized epidermis over its single layer of basal layer [19, 29].
Another growth factor needed for epidermal development is keratinocyte growth factor (KGF), a paracrine factor produced by the fibroblasts of the underlying dermis. KGF is received by the basal cells of the epidermis and probably regulates their proliferation. If the gene encoding KGF is fused with keratin 14 promoter, the KGF becomes an autocrine factor in the transgenic mice. These mice have a thickened epidermis, baggy skin, too many basal cells, and no hair follicles, not even whisker follicles [19, 30].
Many studies have demonstrated that the dermis provides the initial signals required for epidermal specification [16]. In vertebrates, the acquisition of the epidermal fate is associated with the induction of p63 expression, which is the first transcription factor to be specified for the epidermal lineage [16, 31–36]. It has been demonstrated that mice that are deficient for p63 gene function have truncated or absent limbs, poorly developed skin, and die shortly after birth, presumably due to dehydration [13, 37, 38]. p63 is involved in the development of the embryonic basal layer (Fig. 1.4a). In the epidermis, at least six p63 isoforms are expressed, which fall into two categories: those that encode proteins with an amino-terminal transactivation domain (TA isoforms) and those that encode proteins that lack this domain (ΔN isoforms) [13]. Among the TA or ΔN isoforms, alternative splicing gives rise to three different carboxyl termini that are associated with the designations of α, β, and γ [13, 39]. Regulation by p63 involves an intricate interplay between various p63 isoforms [13, 20]. Specifically, TAp63 isoforms are detected prior to the commitment to stratify and strongly localize to the nucleus, indicating that they may be required to initiate the epithelial stratification program in the developing embryo [13]. Particularly, the TAp63α isoform induces expression of AP-2γ, a transcription factor implicated in the regulation of K5 and K14 expression during epidermal morphogenesis [6, 16, 23, 36, 40–43]. Other than K14 expression, the epidermal fate is also associated to the expression of desmosomal components, important for cell-cell adhesion within the epidermis [18, 44] (Fig. 1.4a). One desmosomal component directly involved by p63 in the embryonic basal layer is Perp [6, 16, 45]. Moreover p63 is important for regulating the adhesion of keratinocytes to the basement membrane which is mediated by integrins, a family of transmembrane receptors for the basement membrane protein laminin [6, 46]. Specifically, it has been demonstrated that p63 induces expression of several integrin subunits, such as integrin α3 [6, 47, 48]. Furthermore, p63 controls basement membrane formation by directly inducing the expression of the basement membrane component Fras1 [6, 18].
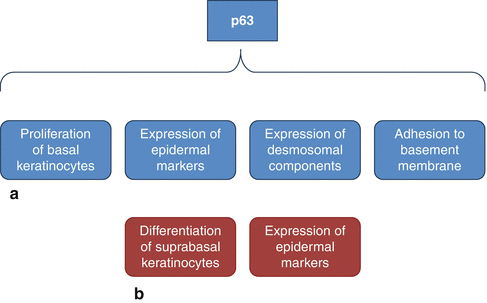

Fig. 1.4
Roles of p63 in the development of the epidermis. (a) p63 is involved in several processes important for the formation of the embryonic basal layer; (b) p63 isoforms also control terminal differentiation of keratinocytes (See more details in the text)
The basal layer other producing keratinocytes that undergo terminal differentiation provides the epidermis with mechanical stability and barrier function (Fig. 1.4b). To this purpose, some keratinocytes terminally differentiated are ultimately sloughed off, whereas other cells which can continuously supply terminally differentiating keratinocytes must be maintained for the life of the organism [16]. In order to prevent premature terminal differentiation, basal keratinocytes must repress the expression of genes that initiate this process and, at the same time, must induce and maintain the expression of genes required for proliferation and K5/K14 expression [16]. It has been demonstrated that ΔNp63α can directly induce K14 expression, and it is probably involved in maintenance of K14 expression in keratinocytes [16, 43, 49]. Moreover, ΔNp63α is able to maintain keratinocyte proliferation by directly inhibiting the expression of two genes induced during epidermal terminal differentiation: p21WAF/Cip1, a member of the cyclin-dependent kinase inhibitor (CKI) family [13, 50], and 14-3-3σ, a member of 14-3-3 family of intracellular signaling proteins [13, 16, 51–57]. ΔNp63α binds directly to the p21WAF/Cip1 and 14-3-3σ promoters [55], so inhibiting their transcription and then favoring epidermal cell proliferation [13]. In addition, ΔNp63α inhibits p21 expression by preventing Notch signaling, an upstream regulator of p21 in the epidermis [16, 57–59]. Moreover, it has been hypothesized that Notch signaling is involved not only in growth arrest but also in cornification [13]. In fact, it has been demonstrated that addition of JAG-1 to human keratinocytes lift cultures resulted in Notch activation; strong induction of loricrin, involucrin, and peroxisome proliferator-activated receptor-γ (PPARγ); and cornified envelope formation [13, 60] other than in enhanced levels of nuclear NF-κB [13, 61]. Furthermore, p63 also represses two cell cycle inhibitors Ink4a and Arf [6, 62] as well as induces the expression of genes required for cell cycle progression, including ADA and FASN [6, 55, 62–66]. Ink4a regulates cell cycle arrest by blocking phosphorylation of Rb family members, thereby maintaining them in their anti- proliferative states [20, 67]. On the other hand, it has been demonstrated that p63 is able to directly repress the expression of genes required for cell cycle progression including cyclin B2 and cdc2 [6, 16, 68]. This apparent controversy may be explained by supposing that p63 functions to maintain proliferation in early transit amplifying (TA) cells, whereas it acts inducing cell cycle exit in mature TA cells [6, 16]. In fact, it has been demonstrated that p63 directly induces p57Kip2, a cyclin-dependent kinase inhibitor, in order to allow cell cycle exit and undergo terminal differentiation [6, 69, 70]. Specifically, whereas TAp63 isoforms inhibit terminal differentiation [36], ΔNp63 isoforms are first expressed after the single-layered epidermis has committed to stratification [13]. In vivo studies using transgenic mice suggest that one function of ΔNp63α during early epidermal morphogenesis is to counterbalance the effects of TAp63-induced inhibition of differentiation, allowing cells to respond to terminal differentiation program [13, 36]. ΔNp63α may block TAp63 isoforms directly, via a dominant-negative action of TAp63 [13]. As reported for p63 and Notch, another molecule involved in the switch from proliferation to growth arrest is NF-κB [13, 71]. In normal epidermis, NF-κB proteins localize in the cytoplasm of basal cells and then in the nuclei of suprabasal cells [13, 71]. Particularly, it has been shown that NF-κB in association with selective induction of p21WAF/Cip1 induces growth arrest [13, 72]. As p63, also NF-κB acts by downregulating molecules that promote cell proliferation [13]. Specifically, during epidermal development, NF-κB functions by opposing the proliferative activity of TNFR1/JNK [13].
In humans, the earliest morphological sign of stratification during epidermal development is the formation of the intermediate cell layer [14, 16, 73]. The intermediate keratinocytes, which proliferate and express K1, populate the first suprabasal layer of the embryonic epidermis [14–16, 18]. ΔNp63α is the only transcription factor known to be required for the formation of the intermediate layer [16]. In fact, ΔNp63α synergizes with Notch to induce K1 expression [16, 57].
At the same time, it has been shown that ΔNp63α expression is reduced to approximately 25 % in suprabasal keratinocytes with respect to its high expression in basal keratinocytes [6, 74]. Its rapid downregulation in suprabasal keratinocytes is mediated by several processes: first, ΔNp63α transcripts are degraded by microRNA-203 [6, 75, 76] which is expressed only in suprabasal keratinocytes [6, 75–77]; second, ΔNp63α protein is also actively degraded in suprabasal keratinocytes through the proteosomal pathways by the E3 ubiquitin ligase Itch and p14Arf [6, 78, 79].
The intermediate cell layer exists only transiently, since intermediate cells are replaced by postmitotic keratinocytes that form spinous layer during later developmental stages [14, 16]. Histological analysis has demonstrated that intermediate cells mature directly into spinous cells [16, 18, 73]. Despite ΔNp63α active degradation in suprabasal cell layers, the remaining protein is sufficient to control important aspects of keratinocytes differentiation and particularly seems to be involved also in this process. In fact, ΔNp63α critical target gene is IKKα [18], a previously identified regulator of epidermal, skeletal, and craniofacial morphogenesis [16, 80–82] which is a critical mediator of cell cycle exit during keratinocyte differentiation [6, 18, 83]. Intriguingly, the failure of intermediate cells to mature into spinous cells, lacking IKKα, also resulted in the aborted development of epithelial appendages and limbs [16, 84].
An important trigger of epidermal terminal differentiation is an increase in extracellular Ca2+ concentration, which is involved in regulating the formation of the spinous layer, granular layer, and epidermal barrier [16]. A Ca2+ gradient is first established in utero, and in mature epidermis, an increasing gradient of extracellular Ca2+ is present from basal to the cornified layers [16, 85–87]. Specifically, protein kinase C (PKC) is activated during keratinocyte differentiation [16, 88]. During terminal differentiation, PKC proteins appear to function specifically in the transition from spinous to granular cells, by contributing to downregulate K1 and K10 expression [16, 89], a process that is associated with the transition from spinous to granular cells [27, 90]. In addition, PKC activation induces expression of loricrin, filaggrin, and transglutaminase, markers of granular keratinocytes [16, 89, 91].
The final step in epidermal stratification involves the formation of the epidermal barrier [16]. The best-studied transcription factor involved in the regulation of epidermal barrier formation is Klf4 which is expressed in the upper spinous and granular layers [16, 92, 93]. Another transcription factor implicated in this process is Grhl3/Get1 [94, 95] which downregulates many genes involved in lipid synthesis and metabolism [16]. Finally, it has been demonstrated that ΔNp63α is also important for the formation of the epidermal barrier by inducing at least two genes required for barrier formation: Claudin 1 and Alox12 [6, 96, 97]. Moreover, several in vivo and in vitro studies indicated that PPAR activators accelerate differentiation and cornification in fetal and adult epidermis [98–101]. It has been demonstrated that Notch signaling may be involved also in these processes. In fact, Notch may function upstream of PPARs to induce terminal differentiation [13]. Moreover, Notch also induces the caspase 3 in order to promote terminal differentiation during embryonic development of epidermis [13].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree