The promise of personalized therapies for breast cancer can only be fulfilled through tissue-based correlative science studies. Unfortunately, access to a tissue bank has been the major barrier to the development of prognostic or predictive tests. This chapter describes issues related to tissue acquisition and banking for breast cancer in the multicenter clinical trial setting.
Although survival outcome of patients has improved greatly over the past 2 decades due to incremental benefits achieved from systemic therapies, it has become quite clear that molecular heterogeneity of breast cancer dictates treatment response and not every patient derives significant benefit from a given therapy.1,2 Development of assays that enable assessment of baseline risk and expected degree of benefit from systemic therapy in specific clinical contexts has become a top priority in the research agenda for breast cancer.
These kinds of correlative science studies need tumor tissue with good clinical annotation. Best source of such materials is from randomized phase 3 clinical trials. Unfortunately, tissue procurement is most difficult in such a setting. Ideally every single tumor operated should be snap frozen according to a uniform standard operating procedure and archived in a proper environmental condition. However, reality is that in a typical practice setting in North America, procurement of snap frozen biopsy tissue in expectation of future correlative science studies is often difficult, if not impossible. Because patients usually are presented with the option of enrolling into a clinical trial only after cancer diagnosis is made, the window of opportunity to procure snap frozen tumor tissue often gets lost. National infrastructure to procure snap frozen tissue samples from all tumors operated and store in an ideal environment before shipping to a central bank does not exist, and liquid nitrogen or dry ice may not be readily available in a community oncology setting. For this reason, frozen tissue procurement in large phase 3 multicenter adjuvant clinical trials has not been successful.
Surprisingly, even procurement of formalin-fixed paraffin-embedded tumor blocks has been lacking in phase 3 adjuvant clinical trials until only recently, which is now mandated by many clinical trial groups. Although the utility of archived formalin-fixed paraffin-embedded tumor tissue has been limited by technological barriers, recent development of gene expression analysis methods enabled National Surgical Adjuvant Breast and Bowel Project (NASBP) investigators to use these nonideal materials from already completed trials for the development of the OncotypeDx assay (Genomic Health Inc., Redwood City, California), which is now widely used in the prognostication of outcome in patients with estrogen receptor positive, node-negative breast cancer.1,3 Therefore there is now a clear scientific rationale to mandate tumor block collection in all clinical trials.
Most molecular profiling methods dictate the importance of using snap frozen tissue as the starting material. This has especially been the norm for gene expression profiling studies using microarrays. Ideally in an academic setting with active clinical investigation, dedicated personnel such as pathology assistants should be on call to procure specimens immediately after they were removed from patients. A staff pathologist should be available to grossly examine the specimens, to triage them according to study and banking requirements, and to aliquot them into various fixing and storage conditions. How much time lapse can be tolerated between the removal of tissue from the body and freezing is still the subject of debate. There are few published studies that addressed the impact of time delay on RNA integrity. For normal breast tissue, up to 3 hours was tolerable in one study.4
There are now methods that allow gene expression profiling of archived formalin-fixed paraffin-embedded blocks, but proteomics studies still require frozen tumor tissue.5 How much time lapse can be tolerated for proteomics studies is not clear. It is generally believed that immediate freezing is required for studies interrogating the phosphorylation status of proteins.
In a community setting with lack of infrastructure, one alternative to snap freezing is the use of the RNAlater solution (Applied Biosystems; Ambion, Austin, Texas).6 This is a high-salt solution that enables procurement of tumor tissue at room temperature and shipping and storage at 4°C before going into a permanent storage at -20°C. Therefore, RNAlater can be used at institutions where deep freezers or liquid nitrogen tanks are not available for long-term storage of frozen specimens. RNAlater is also useful in a multicenter trial setting when frozen tumor tissue needs to be procured at local sites and shipped to the central bank. Studies demonstrated that tumor tissue specimens procured and stored in RNAlater can be used for microarray gene expression analyses.6,7 However, in one study using colon and tonsil tissue, RNAlater was found to impact on expression level of a gene, whereas simple transportation in ice preserved expression levels up to 16 hours.8 Because for breast tissue up to 3 hours at room temperature is tolerated,4 it may be a good idea not to use RNAlater purely for the purpose of preventing RNA degradation during time delay between surgical procedure and freezing.
In one of the trials conducted by the NSABP, 66 of 76 core biopsies (88%) procured and shipped using RNAlater yielded high-quality RNA adequate for microarray gene expression analyses.9 In NSABP neoadjuvant trials, RNAlater is routinely used to procure and ship core biopsy specimens. Figure 40-1 shows the components of a custom-made RNAlater shipping package. It includes two polypropylene bottles containing 5 mL of RNAlater solution, blotting papers, vinyl gloves, freezer pack, and instructions on how to handle the specimen and package it into a plastic foam and corrugated box, compliant with transportation regulations.
Figure 40-1
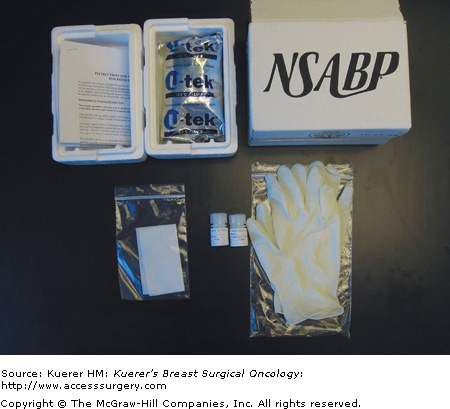
Components of the RNAlater tissue procurement and shipping kit used in the NSABP trials. It includes 2 polypropylene bottles containing 5 mL of RNAlater solution, blotting papers, vinyl gloves, freezer pack, and instructions on how to handle the specimen and package it into a plastic foam and corrugated box compliant with transportation regulations.
One disadvantage of using RNAlater is that tumor tissue becomes too hardened and, as a result, preparing cryosections from such tissue is very difficult. One solution to this problem is to wash the tissue in saline before cutting6 or blot dry the tissue to remove excess RNAlater before embedding and cut the tissue at below -20°C.9 After cutting a few sections for microscopic examination and assessment of tumor cellularity, the tumor-rich area can be manually dissected and homogenized immediately or stored in RNAlaterICE solution (Applied Biosystems) at -20°C before batch processing to extract RNA.
RNAlater can be also used to prevent RNA degradation during the processing of the cryosections from archived frozen tumors that were not preserved in RNAlater.10
Although RNAlater preserves RNA and DNA, some proteins such as S-100 protein do get affected by this high-salt solution, and therefore RNAlater is not recommended for proteomics studies.11
Difficulties of collecting frozen tumors from adjuvant trials and sample size considerations have resulted in a proliferation of neoadjuvant trials as a biomarker research tool in recent years.12 Neoadjuvant trials provide a unique setting, in which surgical oncologists can control the acquisition of pretreatment tumor tissue samples and the molecular profile of these samples can be correlated with response to preoperative therapy. Pathologic complete response (pCR) to neoadjuvant therapy eventually correlates with clinical outcome and thus provides a convenient alternative to long-term follow-up. Because the same statistical power can be achieved with much smaller sample sizes than in the adjuvant setting, many clinical investigators favor neoadjuvant trials over adjuvant trials as a correlative science research platform. However, for high-throughput discovery approaches such as gene expression microarrays or proteomics, the typical sample sizes of neoadjuvant trials are grossly underpowered when the issue of multiple comparisons is considered. Furthermore, neoadjuvant trials do not consider the effect of additional adjuvant therapies such as tamoxifen on baseline risk because only response is measured. Therefore, the clinical utility of markers discovered in the neoadjuvant trials needs eventually to be tested in the adjuvant setting. Because the predictive algorithm for predicting pCR, developed in the neoadjuvant setting, cannot be directly applied into the adjuvant setting, where time-dependent end points are analyzed, it may make more sense to use materials from adjuvant trials even for the discovery step. The fact that the only multigene-based predictor of chemotherapy benefit in clinical use (OncotypeDx) was developed purely through use of archived tumor blocks from adjuvant trials attest to the latter point.
Procurement of Formalin-Fixed Paraffin-Embedded Tumor Tissue Blocks
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







