Thyroid Hormone Transport Proteins and the Physiology of Hormone Binding
Salvatore Benvenga
Thyroid hormones (TH) are low molecular weight molecules that signal to virtually all tissues, predominantly by a slow genomic effect on a multitude of genes after binding to a variety of TH nuclear receptors (THR) but also by rapid non-genomic pathways and even as neurotransmitters co-released with norepinephrine. This pleiotropy of modes of action on target tissues, target genes, THR, as well as TH-binding sites on the cell plasma membrane for TH entry and exit, is associated with a multitude of plasma transport proteins. This transport is unavoidable because, similar to steroid hormones, TH are hydrophobic.
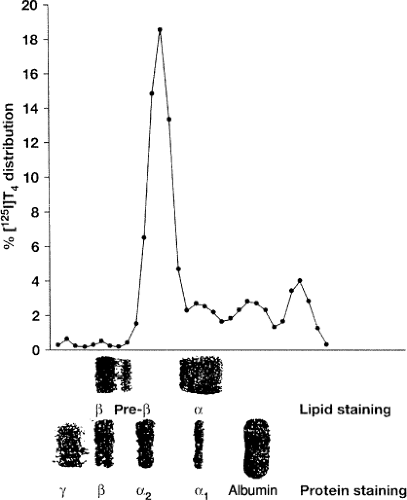
There are three conventional major carriers for TH (Fig. 6.1) [thyroxine-binding globulin (TBG), transthyretin (TTR), and albumin (HSA) (Table 6.1)], in addition to a number of minor carriers (1) (Table 6.2). Some carriers are also sterol hormone–binding proteins (SHBP). For example, cortisol has cortisol-binding globulin (CBG) as a major carrier, and HSA and sex hormone–binding globulin (SHBG) as minor carriers (2). The opposite is true for dihydrotestosterone, whereas estradiol (E2) has both HSA and SHBG as a major and CBG as a minor carrier (2). Of the total circulating 1,25(OH)2D3 and less potent 25(OH)D, 85% and 90% are bound to the α2-globulin vitamin D–binding protein (VDBP) and the remainder to HSA, a VDBP homologue (3). However, about half of the intestinal absorbed vitamin D is carried by lipoproteins (Lp) (4).
Table 6.1 Main Characteristics of the Major Protein Transporters of Thyroid Hormones in Humans | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 6.2 Minor Thyroid Hormone Plasma Carriers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TBG and CBG are non-inhibitor members of the family of the serine protease inhibitors (serpins). In human plasma, the free fraction of TH [∼0.03% of total T4 and ∼0.3% of the biologically more potent T3 is lower than free steroid hormones (>1%), similar to the free fraction of 25(OH)D (0.03%) and the more potent 1,25(OH)2D3 (0.4%). To add to the parallelism between TH and vitamin D, VDBP in the Emydidae family of turtles is a high-affinity binder of both vitamin D and T4 (5). Finally, considering that one TBG homologue, TTR and Lp are carriers of retinoids (or the retinoid precursor retinol), then it is of interest that such diverse hormones, which share the ancestors of the corresponding nuclear receptors, also share a number of plasma carriers. Indeed, nine hydrophobic repeats in domain 3 of α-fetoprotein (AFP) – a homologue of HSA and VDBP that binds TH, steroid hormones, and retinoids (6,7) – have structural homology with the heptad dimerization repeats of the nuclear receptor superfamily (8).
A survey of the expression of HSA, TTR, and TBG genes in various species during development provides clues as to how the present TH distribution network in extracellular compartments developed during vertebrate evolution (9). HSA may be the oldest and TTR is the second component of the network. The addition of TBG occurred at postnatal stages in some marsupials and rodents, and in perinatal to adult stages in most eutherians. Appearance of TTR and TBG may be related to the increased requirement of TH for TH-dependent tissue remodeling during developmental stages and/or increased metabolism in TH-target tissues with the acquisition of homeothermy (9). For compounds, including environmental pollutants (10,11,12,13,14), affecting TH binding to plasma carriers, see Chapter 57.
Thyroxine-Binding Globulin (TBG)
TBG (SerpinA7) is the major plasma carrier of TH in most of the large mammals. Of 100,000 molecules of circulating TBG, approximately 20,000 are occupied by T4 as compared to approximately 300 of TTR and 3 of HSA. Unlike TTR and HSA, TBG is a glycoprotein. The four carbohydrate chains contain the following oligosaccharide residues per TBG molecule: 10 of sialic acid, 9 to 13 each of galactose and mannose, and about 20 of glucosamine. The carbohydrate moiety contributes, with TH, to its molecular stability and is responsible for TBG microheterogeneity in the pI (isoelectric point) 4.2 to 5.0 range (the greater the sialic acid content, the greater the anodal mobility). Zhou et al. (15) reported the 3D structure, solved at 2.8 Å, of human non-glycosylated TBG complexed with T4. T4 is bound in a pocket between helices H and A and strands 3 to 5 of sheet B. T4 is held in the TBG pocket by a series of hydrophobic interactions with underlying residues and by hydrogen bonding of the aminopropionate of T4 with adjacent residues. T4 is oriented in a cisoid conformation, with the aminopropionate and outer phenolic ring both on the same side of the inner ring of the molecule, as in the crystal structure of the HSA–T4 complex. TBG strikingly differs from other serpins in having the upper half of its main sheet (sheet A) fully opened, so its reactive center peptide loop can readily move in and out of the sheet to give an equilibrated binding and release of T4. The entry of the loop triggers a conformational change, with a linked contraction of the binding pocket and release of the bound T4. Thus, TBG has adapted the serpin inhibitory mechanism to give a reversible flip-flop transition, from a high-affinity to a low-affinity form. The complexity and ready triggering of this conformational mechanism strongly indicates that TBG has evolved to allow a modulated and targeted delivery of T4 to the tissues (15). Segments 215 to 291 and 365 to 395 are the part of human TBG most conserved in mammal TBG (>95% similarity) (16), and indeed, the acid dissociation constant (Ka) for T4 of mammal and human TBG match (16).
The large discrepancy between zone electrophoresis (ZE) and radioimmunoprecipitation (RIP) (Table 6.1) is seen because TBG migration overlaps with other α-globulins, whereas TTR and HSA do not comigrate with other proteins. At an average concentration of 2 mg per dL in a serum containing 7 g per dL total protein and 0.98 g per dL total α-globulins, TBG accounts for only 0.2% of the total plasma α-globulins. Thus, a number of other α-globulins could bind TH and be confused with TBG in ZE. This is the case of high-density lipoproteins (HDLs), the α-migrating Lp (Fig. 6.1), which bind approximately 3% of circulating T4. Other binders are the TBG homologues α1-AT (SerpinA1), α1-chymotrypsin (α1-ACT or Serpin A3), antithrombin III (AT-III or SerpinC1), and CBG (SerpinA6), and the non-homologous α1-acid glycoprotein (AGP) and SHBG, a β-globulin (1) (Table 6.2). Additional T4 carriers could include AFP (6,7,8) and lipocalins (see section Additional Proteins). Serpins have one TH site with relative affinity T4 > T3, but Ka for T4 is much lower than in TBG (1). Similar to TTR (see later), expressed in the ependymal cells of the choroid plexus, but primarily in neurons of the adult brain, is the TBG homologue neuroserpin (Serpin1). Variants of this axonal-secreted protein cause an autosomal-dominant form of dementia (17). Neuroserpin is likely to be a low-affinity TH carrier, and impairment of TH binding could favor the intraneuronal polymerization of neuroserpin (18).
The binding of E2, progesterone, testosterone, cortisol, aldosterone, and all-trans-retinoic acid to the inhibitory serpins AT-III, heparin cofactor II, plasminogen activator inhibitor I, and protein C inhibitor (PCI) was studied. Only all-trans-retinoic acid bound to PCI (fivefold more than buffer vs. twofold for the other hormones), specifically with a dissociation constant (Kd) of 2.4 μM and one site per mole (19). All trans-retinoic acid is also bound by plasma Lp (20).
Transthyretin (TTR)
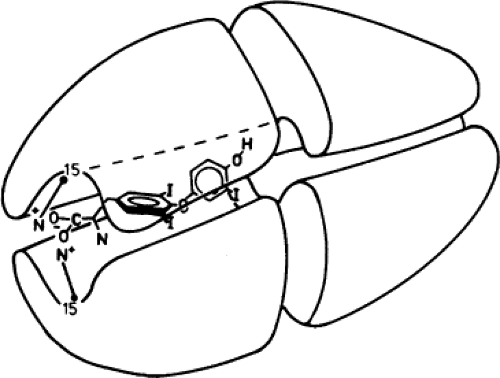
TTR circulates as a homotetramer of known crystallographic structure. The four subunits are arranged symmetrically around a cylindrical channel (Fig. 6.2). The central β-barrel where TH binds is formed by eight strands (strands A–H, corresponding to amino acids 11 to 19, 29 to 32, 42 to 49, 53 to 55, 67 to 74, 91 to 97, 105 to 112, and 115 to 121). Strands G and H contribute 6/10 residues of the TH-binding surface. The TH sites of the homotetramer are two, but only one is occupied due to the much lower Ka of the second site, as a result of negative cooperativity. Crystallography studies have shed light on the structural basis for negative cooperativity (21). The primary site has a larger diameter than the second site, and T4 (or other ligand) binding is followed by a slight collapse of the
outer and inner parts of this site, and concomitant opening of the second site. Binding of the second ligand is then followed by collapse of the second site. However, this second collapse is more limited, so that the second molecule of ligand is bound less tightly. Recently, the crystal structure of human TTR complexed to two TH synthetic analogs, GC-1 and GC-24, was determined (22).
outer and inner parts of this site, and concomitant opening of the second site. Binding of the second ligand is then followed by collapse of the second site. However, this second collapse is more limited, so that the second molecule of ligand is bound less tightly. Recently, the crystal structure of human TTR complexed to two TH synthetic analogs, GC-1 and GC-24, was determined (22).
TTR is predominantly a retinol-binding protein (RBP), because RBP is carried by one in three molecules of TTR, and there are four RBP-binding sites, but only one is likely to be occupied. The RBP and TH sites are independent. RBP, in turn, binds retinol (vitamin A) in a 1:1 molar ratio. Retinol, retinoids, carotenoids, and 1% to 2% of TTR itself are carried by Lp (20,23,24,25).
TTR is the sole member of its family, a gene of much more ancient lineage than TBG. Indeed, TTR is present in marsupials, rodents, Insectivora, birds, reptiles, amphibians, and fish, and open-reading frames for TTR gene–like nucleotide sequences were found in bacteria, yeast, and the nematode Cunninghamella elegans (26). Of interest, TTR of birds, reptiles, amphibians, and fish binds T3 preferentially (26). The switch to the preferential binding of T4 in mammals was due to selective pressure on the N-terminus of TTR, which became shorter and more hydrophilic during evolution (26). Similar to TBG and apolipoproteins (apos), the T4-binding domain is the part best conserved in the phylum. In nonhuman animals, plasma TTR binds more TH than does TBG, but regardless of species, TTR is the major TH transport protein in the cerebrospinal fluid (CSF) (26). The vast majority of TTR in CSF derives from central nervous system synthesis (26). This synthesis (which is restricted to the choroid plexus, except for very little in the meninges) is absent in amphibians and fish, namely in species without a neocortex, but it does exist in species as ancient as reptiles, which are the first species showing traces of a cortex (26). TTR has neuroprotective actions, centrally and peripherally, which require megalin-mediated cell internalization of TTR (27). TTR was also detected in the vaginal fluid of rabbits, and it was one of the proteins of that fluid that exerted antimicrobial effects against gram-negative and gram-positive bacteria (28). Expression of both TTR and megalin was detected in human scalp skin and hair follicles (29). Placenta is another site of synthesis of TTR, this tissue being also capable of internalizing the T4–TTR complex (30,31).
Local amino acid sequence homology exists between the tail of TTR (amino acids 90 to 127) and the glucagon–secretin family of gastrointestinal hormones (32), while two TTR segments (amino acids 18 to 53 and 72 to 117) share homology with the amyloid-related proteins (ARP), including the amyloidogenic A4 (or Aβ) segment of the amyloid β-protein precursor (AβPP) associated with Alzheimer’s disease (33). The homology with ARP is relevant, because native TTR and several TTR variants are involved in senile amyloidosis and familial amyloid polyneuropathy, respectively. Finally, amino acids 38 to 57 of the β-subunit of human luteinizing hormone (LH) are homologous with residues 10 to 30 of TTR (34).
Albumin (HSA)
This ancient multiligand protein consists of three repeated domains (amino acids 25 to 205, 212 to 397, and 404 to 595 in humans), each containing two subdomains (A and B). By crystallographic analysis, four T4 sites were identified in subdomains 2A, 3A, and 3B (35). A fifth site is in the cleft between domains 1 and 2, created by the conformational changes on HSA induced by binding of the fatty acids (36). Fatty acids inhibit TH binding to plasma proteins; on HSA they bind in hydrophobic pockets that are distributed asymmetrically (36). Important for the high affinity of the site in subdomain 2A is Arg218. As mentioned above, HSA binds sterol-derived hormones, and its homologue VDBP binds both T4 and vitamin D in turtles of the Emydidae family (6), whereas the homologue AFP binds TH, steroid hormones, and vitamins (6,7,8).
 Figure 6.3 Amino acid sequence homology concerning the thyroid hormone (TH) binding domains of TH transport plasma proteins. This is an update and expansion of the homology between apolipoproteins (apos), thyroxine-binding globulin (TBG), transthyretin (TTR), and albumin (HSA) described in (45). Conserved residues are typed boldface, and position is marked by an asterisk to indicate conservation in at least 60% of the 21 aligned proteins. Confirming the initial data in (45), note the conservation of the hydrophobic motif Y, L/I/M/, X, X, V/L/I. Also, note the emergence of the longer motif E/Q/N/D, V/L/I, X1–X33, Y,L/I/M/, X, X, V/L/I. The four underlined residues of SHBG interact with steroid hormones, and a fifth one (glycine) is just N-terminal to the threonine in the first position of the alignment. The homology between TBG, TTR and HSS is longer (not shown). The homologous sequences are amino acids 292 to 348 and 497 to 534 of HSA, 72 to 122 of TTR (a segment that contains 6 of the 10 residues that form the TH-binding surface, and strands F, G, and H of the binding channel), and amino acids 231 to 273 and 349 to 395 of TBG, corresponding to β-strands B2, B3, B4, and C2. |
Lipoproteins (Lp)
Gel filtration chromatography (GFC) of human plasma demonstrated binding of T4 (∼3%), T3 (∼6%), and reverse T3 (rT3) (∼0.2%) to all classes of Lp: Chylomicrons, very low-density Lp (VLDLs), low-density Lp (LDLs), and high-density Lp (HDLs) (37,38) (Table 6.2). However, there might be dissociation of TH from Lp during GFC, because ZE and RIP give higher binding values (38). Distribution of TH between the numerous HDL subfractions is affected by thyroid status (39,40). Interestingly, one HDL particle has the same molecular weight as HSA, has very little lipid, and its only protein moiety is apoA-1 (41). The association of TH with the lipid moiety of Lp has not been quantified. TH, though, bind specifically to apoA-I, apoA-II, apoA-IV, apoB-100, apoC-I, apoC-II, apoC-III, and apoE (37,38,42,43,44). The Ka for TH of the Lp (i.e., the lipid-associated apos) is one to two orders of magnitude lower, because lipids (including fatty acids) inhibit TH binding to apos (45). Chemical pollutants also affects TH binding to Lp (46).
The non–apoB-100 apos contain a single TH site of 107 M-1 binding affinity (37,44), with relative affinity T4 =rT3 > T3. The TH site is N-terminal, in the exon 3-coded region (exon 2 for apoA-IV), which contains β-sheet structure. There is remarkable local homology in the TH-binding domains of apos, TBG, TTR, HSA (44), and other carriers (Fig. 6.3). Apos share the hydrophobic motif Y, L/I/M/, X, X, V/L/I, the first two residues being conserved in TBG, TTR, and HSA. Particularly, Y matches F249 of TBG. This motif is well
conserved in the phyla, and it is believed to represent the core of the TH-binding site (43,45). ApoB-100 contains three TH sites, one in each of three non-overlapping fragments generated from the natural cleavage by circulating enzymes (42). Experimental data indicated TH sites at amino acids 380 to 392, 1,310 to 1,338, and 4,223 to 4,510 (42), in keeping with the sequences 360 to 406, 1,303 to 1,344, and 4,281 to 4,341 found by homology search (43).
conserved in the phyla, and it is believed to represent the core of the TH-binding site (43,45). ApoB-100 contains three TH sites, one in each of three non-overlapping fragments generated from the natural cleavage by circulating enzymes (42). Experimental data indicated TH sites at amino acids 380 to 392, 1,310 to 1,338, and 4,223 to 4,510 (42), in keeping with the sequences 360 to 406, 1,303 to 1,344, and 4,281 to 4,341 found by homology search (43).
In the trout, Lp (HDL > LDL > VLDL) are the major T4 and T3 carriers (47), but unlike human Lp, the Ka values for T4 and T3 are similar (47). This recalls the evolutionary changes in TH affinity of TTR (26). Lp also bind the lipid-soluble vitamins (4,23,48) and steroids (49) (S. Benvenga, unpublished observation). Fish eggs contain carotenoids, retinals (retinal and dehydroretinal) and retinols (retinol, dehydroretinol and retinyl-esters) that are utilized during embryonic development, after fertilization (25). The carotenoids (mainly astaxanthin) are transported in the plasma by LDL, HDL, very high-density Lp (VHDL), and were found to be also associated with HSA. Retinals were found to be associated with vitellogenin (VTG), a component of the plasma VHDL that is internalized by oocytes during vitellogenesis (25).
Immunoglobulins
Immunoglobulins (Igs) are minor TH carriers (Table 6.2 and Fig. 6.1). Unlike ZE, RIP permits discrimination between Ig classes (50,51). Above each one’s laboratory cutoff point (i.e., the greatest proportion of TH bound to any Ig class in healthy persons), the serum is said to contain TH autoantibodies (THAbs). These can be T3Ab, T4Ab, or T3 plus T4Ab (approximately half, one-fourth, or one-fourth of the cases, respectively). Traditionally, THAbs are considered to be IgG, though other Ig classes were not always investigated. Cases of IgA, IgM (52), and IgE THAbs were reported (53). In one patient, THAbs also bound steroids (52,54).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree