Fig. 1
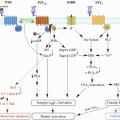
Mechanism of action of unfractionated heparin, low-molecular-weight heparin, and fondaparinux
Only one-third of UFH molecule contain the high-affinity pentasaccharide required for anticoagulant effect [22]. The UFH fraction with low-affinity for AT have more effect on platelet function [23]. In vitro, UFH binds to platelets and, based on experiments, can either induce or inhibit platelet aggregation [24]. In vivo, UFH can prolong the bleeding time in humans [25] and can increase vessel wall permeability in rabbits [26]. The interaction of UFH with platelets can lead to heparin-induced bleeding through mechanisms independent of its anticoagulant effect [27].
Besides its hemorrhagic adverse effects, UFH inhibits the proliferation of vascular smooth muscle cells [28], and promotes osteoporosis through inhibiting osteoblast formation and activation of osteoclast [29]. Heparin-induced thrombocytopenia (HIT) is the most common non-hemorrhagic adverse effect of UFH [15].
4.1.2 Pharmacokinetics
UFH has marginal intestinal absorption and for that reason must be administered parenterally [20]. UFH is given by an initial bolus dose, followed by continuous infusion. If patient intravenous line is not readily accessible, weight adjusted subcutaneous UFH may be a suitable choice.
There is inter-patient variability to the anticoagulation response to UFH because after entering the circulation, UFH binds to plasma proteins different from AT, which can reduce its bioavailability [20].
Heparin is eliminated by a combination of a rapid saturable, dose-dependent mechanism and a slower, nonsaturable, dose-independent mechanism. At therapeutic doses, a large proportion of UFH is eliminated rapidly from plasma by binding to endothelial cells and macrophages [8]. No clear recommendations is available for reducing the maintenance dose in CKD patients. However, high doses of UFH are eliminated predominantly by the slower nonsaturable mechanism of renal clearance once saturation of cellular mechanism occurs. Therefore, over anticoagulation can occur in patients with severe CKD (GFR <30 mL/min/1.73 m2), and the dose may need reduction to avoid the risk of bleeding [13].
4.1.3 Dosing
In acute thromboembolic events, an intravenous bolus of up to 80 units/kg and a continuous infusion of 18 units/kg/h may be administered to rapidly reduce the activity of clotting factors [13]. In the setting of acute coronary syndromes (ACSs), a lower intravenous bolus dose of 60 units/kg and 12 units/kg/h is commonly used [30].
In the absence of acute need for anticoagulation or in the case of high bleeding risk, the bolus dose may be avoided and only a continuous infusion may be initiated. This has the benefit of establishing more steady anticoagulation therapy, reducing the risk of bleeding and removing the need for early monitoring of activated partial thromboplastin time (aPTT) used in the case of bolus dose. The target aPTT ranges used in the initial continuous infusion of UFH should be selected based on the indication for anticoagulation therapy and do not require special dose adjustment because of CKD alone [31].
4.1.4 Monitoring
Since the anticoagulation response to UFH varies between patients, it is common practice to monitor UFH and to adjust the dose based on the results of a coagulation test. A retrospective study done among VTE patients in the 1970s reported that an aPTT range between 1.5 and 2.5 was associated with a reduced risk of recurrent VTE [32]. According to this study, a therapeutic range of 1.5–2.5 times baseline gained acceptance [20]. In the setting of ACSs, a lower target aPTT range of 1.5–2 times baseline is desired [30].
4.1.5 Clinical Practice
UFH has been extensively used clinically since 1937, and there are limited randomized clinical trials comparing bleeding complication rates in patients with CKD and those without CKD.
A post-hoc subgroup analysis of the Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-wave Coronary Events (ESSENCE) and Thrombolysis in Myocardial Infarction (TIMI IIB) trials investigated the safety of enoxaparin, a low-molecular-weight heparin (LMWHs), and UFH anticoagulation therapy of ACSs in patients with CKD stages 4–5. Patients were randomized to receive either enoxaparin (unadjusted dose to account for kidney function; 1 mg/kg actual body weight subcutaneously twice daily) and UFH (full-therapeutic dose; intravenous infusion UFH adjusted to maintain an aPTT level of 55–85 s in the ESSENCE and 1.5–2 times control in the TIMI 11B). Although CKD was an exclusion criteria in ESSENCE and TIMI IIB trials, about 2 % of both study population had CKD stage 4 or 5 with no patient on renal replacement therapy. This study showed that the rate of major bleeding in patients with CKD was 6.6 % and in those with no CKD was 1.1 % (P < 0.0001), regardless of whether UFH or enoxaparin was used. However, the number of patients with CKD were small with a total of 143 patients randomized to UFH (n = 74) or enoxaparin (n = 69) compared with those with no CKD (n = 6,969), as was the number of major bleeds among CKD patients (UFH, 4 patients; enoxaparin, 5 patients) [33].
A retrospective medical record review of 620 hospitalized patients with an estimated GFR of less than 60 ml/min/1.73 m2 compared the rates of bleeding in patients who received anticoagulation therapy with full-therapeutic dose of UFH, or with unadjusted enoxaparin (1 mg/kg twice daily). Authors reported that the major bleeding rates were 26.3 per 1,000 person-days for UFH and 20.7 per 1,000 person-days for enoxaparin (nonsignificant), and that the bleeding complications increased for both UFH and enoxaparin therapy at each stage as CKD progressed, suggesting that factors other than drug clearance is responsible for anticoagulant bleeding complications [34].
More recently, a 1 year prospective observational study was conducted in 488 patients with CKD stages 3–5 (estimated GFR, 10–59 ml/min/1.73 m2), who were admitted to the renal ward of a government hospital in Dubai. The study identified the incidence of major bleeding events from therapy with full-therapeutic doses of UFH, or with adjusted enoxaparin (1 mg/kg once daily), and compared the rates of major bleeding between the UFH and enoxaparin users. In this study, major bleeding occurred in 1 in 3 patients who received anticoagulation during hospital stay (hazard ratio [HR], 4.61 [95 % confidence interval [CI], 2.05–10.35]). Compared with enoxaparin users, patients who received anticoagulation with UFH had a higher risk of major bleeding (HR, 4.79 [95 % CI, 1.85–12.36]); with 51 % patients bleeds with UFH versus 22 % patients bleeds with enoxaparin therapy, and had a lower mean [SD] level of platelet counts (139.95 [113] × 103/mL vs 205.56 [123] × 103/mL; P < 0.001) [35].
In the above mentioned studies, the risk of bleeding complication was higher with full-therapeutic doses of UFH compared to enoxaparin. Despite the fact that enoxaparin is dependent on the kidney for its elimination and that it can bioaccumulate with reduced kidney function, this might not result in higher bleeding rates. The increased rate of bleeding observed with UFH may be attributed to the inhibition of platelet function and increase in vascular permeability; properties that are independent to anticoagulant effects [36]. Unlike UFH, enoxaparin binds less to platelets because of its smaller molecular size and so has fewer incidences of heparin-induced thrombocytopenia and bleeding events [36]. This is beneficial because patients with advanced CKD are already more susceptible to bleeding from uremia-related platelet dysfunction [37].
In practice, UFH is used for anticoagulation in patients with high bleeding risks such as patients with CKD, as the anticoagulant effects of UFH can rapidly and completely be reversed by protamine sulphate. The drug formulary recommends that the dose be reduced in patients with severe CKD and patients to be observed carefully for signs and symptoms of bleeding but does not provide any guidance regarding dose adjustment or laboratory monitoring [13].
4.2 Low-Molecular-Weight Heparin
LMWHs are produced from UFH by enzymatic depolymerization. Compared to UFH, LMWHs, when used to treat thromboembolic events, have better pharmacokinetics properties with more favorable efficacy-to-safety ratio [8].
4.2.1 Structure and Mechanism of Action
The molecular weight of a LMWH is about one-third of an UFH, with a mean MW of 5,000 Da, which corresponds to about 15 saccharide units. Each LMWH is produced using different depolymerization methods so there is some variation in their pharmacokinetics properties, anticoagulant effects, and in their dosing strategies [36].
Similar to UFH, LMWHs produce their anticoagulant effect through the AT-mediated inhibition of coagulation factors. UFH chains of at least 18 saccharide units are of enough length to bridge AT to thrombin (IIa), whereas LMWH chains are too short and cannot bind to AT and thrombin (IIa) at the same time although these chains are able to inhibit Xa through AT because the reaction does not need bridging. Compared to UFH, LMWHs specifically inhibit the activity of Xa with lower affinity for thrombin (IIa) (Fig. 1). Depending on molecular size, LMWHs have anti-Xa to anti-IIa ratios from between 2:1 or 4:1. Currently, there is no clinical evidence that the variations in anti-Xa to anti-IIa ratio between different LMWHs affect clinical outcomes such as recurrent thrombosis or bleeding risk [8].
4.2.2 Pharmacokinetics
The pharmacokinetic advantage of LMWH over UFH is that after entering the circulation, LMWH binds less to plasma proteins other than AT, and that it has a bioavailability of around 90 % which leads to more predictable anticoagulant effect than UFH. Also, unlike UFH, LMWH binds less to platelets and PF4 and has a lower incidence of HIT. A disadvantage of LMWH is its dependence on kidney function for excretion and thus accumulation of its anticoagulant effect in patients with decreased kidney function [38]; therefore dosage adjustment is required as kidney function declines.
4.2.3 Dosing
The rates of bleeding with LMWHs differ, with higher rates observed in patients with kidney failure, especially when the dose was not adjusted for kidney dysfunction [39]. For tinzaparin and dalteparin, dosing adjustments are not required when the estimated GFR is more than 20 mL/min/1.73 m2 [16]; However, enoxaparin, because of its smaller molecular size (mean MW 4,400 Da) compared to tinzaparin (mean MW 6,500 Da) and dalteparin (mean MW 5,700 Da), is more depended on kidney clearance requiring a dosing adjustment when the GFR drops below 30 mL/min/1.73 m2 [16].
LMWHs are administered in weight-adjusted doses for both thrombophylaxis or for therapeutic purposes. Numerous studies have helped define dosing adjustment for enoxaparin therapy; these individualized dosing strategies based on weight and kidney function reduce bleeding risk while preserving therapeutic benefits [40]. For example, a pharmacokinetic study in patients enrolled in the TIMI IIA trial [41] suggested that enoxaparin kidney clearance was decreased by 22 % in patients with a CrCl less than 40 mL/min compared with those with greater creatinine clearance (mean, 88 mL/min), which led the authors to extrapolate that enoxaparin clearance would be reduced by approximately 50 % in patients with a CrCl less than 20 mL/min. Authors concluded that patients with a creatinine clearance of less than 40 mL/min had higher trough and peak anti-Xa activity compared with those with no CKD and were more likely to have major bleeding events. Of note in this trial [41], enoxaparin doses were not reduced to account for kidney function that resulted in bleeding events while in a few recent studies [42] it was administrated in adjusted therapeutic doses to CKD patients who were associated with lower bleeding events. The results of these studies [42] highlight the safety of enoxaparin if administered in therapeutic doses with dose adjustment to patients with advanced CKD.
Another theory of reducing the adverse effects of LMWHs in clinical practice is to split their total daily dose into two equal doses, which will reduce trough and peak anti-Xa activity of LMWHs and in accordance minimize bleeding risk associated with these peak in treated patients. Based on a Cochrane review of 22 randomized controlled trials [43] in patients presenting with acute VTE found that the rate of bleeding were similar between the two dosing regimens with the convenience of a once daily dose of anticoagulants outweighing the potential for a lower efficacy. However, the above review was based on the initial treatment of VTE in patients with normal kidney function therefore, due to concerns of over-anticoagulation and subsequent risk of bleeding in patients with decreased kidney function, an initial bolus dose adjustment is needed with monitoring of anti-Xa activity for further individualized dosing strategies.
4.2.4 Monitoring
In clinical practice, it is difficult to measure LMWH concentrations directly therefore, pharmacokinetic studies use surrogate effect markers such as anti-Xa activity, which has been shown to be correlated with the amount of LMWH present, rather than the degree of anticoagulation effect. Some studies have reported that high anti-Xa levels were associated with increased bleeding complications [20]. Thus, routine monitoring of anti-Xa activity is not commonly required during anticoagulation therapy with LMWHs, but may be necessary in patients at increased risk of bleeding such as those suffering from CKD and those who are underweight or overweight [13].
4.2.5 Clinical Practice
LMWHs are used routinely in clinical practice for anticoagulation and have largely replaced the use of UFH. The ease of use, and the predictable anticoagulant effect of LMWHs eliminates the need for routine laboratory monitoring. Many randomized trials have demonstrated the greater safety and clinical efficacy of LMWHs (enoxaparin) compared to UFH in non CKD patients [44]. These large trials excluded CKD patients and the kidney function of randomized subjects was not reported. In general, due to lack of safety and efficacy data from randomized controlled trials, caution is required when administering LMWHs in patients with severe CKD. Prospective studies must be conducted to define the anticoagulant therapeutic activity and dosage adjustments in these patients.
Enoxaparin is the most commonly used LMWHs in patients with CKD. Mostly due to its well studied reduced dose of 1 mg/kg once daily for patients with an eGFR of less than 30 mL/min/1.73 m2 . However, this reduced dose of enoxaparin should not lead to sub-therapeutic anticoagulation therapy in CKD. An investigation conducted among patients with an estimated GFR of less than 30 mL/min/1.73 m2, who received a dose adjusted enoxaparin therapy of 0.61 ± 0.03 mg/kg twice daily showed that under-anticoagulation, defined as peak anti-Xa levels of below 0.5 IU/mL occurred in about 1 in 4 patients; anticoagulation within the therapeutic range, defined as anti-Xa levels from between 0.5 and 1.2 IU/mL which occurred in 1 in 9 patients; and over-anticoagulation, defined as an anti-Xa levels of more than 1.2 IU/mL occurred in 1 in 20 patients [45].
The bleeding risk should be minimized with LMWHs for both the prevention and treatment of VTE in patients with CKD. For example, in a retrospective observational study of 7721 dialysis patients who received thrombophylaxis therapy with either UFH or enoxaparin, reported that enoxaparin was not associated with higher bleeding risk in comparison with UFH (risk ratio, 0.98; 95 % CI 0.78–1.23), concluding that thrombophylaxis doses of enoxaparin appeared to be safe and could be used as an alternative to UFH in dialysis patients [42]. More recently, in a prospective study for the treatment of thromboembolic events in hospitalized patients with CKD from stages 3–5, enoxaparin administrated in adjusted therapeutic doses was associated with lower bleeding events compared to UFH [35]. The results of the study highlighted the safety of enoxaparin when administered in therapeutic doses with dose adjustment for patients with severe CKD.
4.3 Fondaparinux
4.3.1 Structure and Mechanism of Action
Fondaparinux is a synthetic pentasaccharide that is an anti-Xa-specific anticoagulant. It binds to AT and produces a conformational change at the reactive site of AT that increases its affinity for factor Xa. AT then forms covalent bond with factor Xa (Fig. 1). Fondaparinux specific anti-Xa activity is higher than that of LMWHs. Since its molecule is too short to bridge AT to thrombin, fondaparinux does not inhibit thrombin (IIa) through AT [36].
4.3.2 Pharmacokinetics
The pharmacokinetic advantages of fondaparinux over UFH or LMWHs are that it has almost complete bioavailability after subcutaneous injection, has predictable anticoagulant effect, and that it has a long elimination half-life that can be administered subcutaneously one dose daily without routine coagulation monitoring. The disadvantage of its use is that fondaparinux is eliminated by the kidney, therefore it should be avoided in patients with eGFR level of below 20 mL/min/1.73 m2 [13].
4.3.3 Dosing
The appropriate fondaparinux dosage and intervals could range from one dose daily in patients with mild CKD to every other day in more severe CKD settings. For treatment of ACSs or VTE, fondaparinux must be used with caution in eGFR levels of between 30 and 50 mL/min/1.73 m2. For thrombophylaxis, the dose of fondaparinux should be reduced by 50 %, or 1.5 mg once daily if eGFR 20–50 mL/min/1.73 m2 [13]. Numerous studies in patients with moderate CKD (CrCl of 20–50 mL/min) reported that 1.5 mg of fondaparinux given once daily was effective and safe for preventing VTE [46].
4.3.4 Monitoring
Fondaparinux can be monitored by measuring its anti-Xa activity in a way similar to the one used for monitoring LMWHs. Fondaparinux rarely needs monitoring due to its predictable anticoagulant activity, however its dose must be monitored in patients with CKD [20]. In a study that was conducted among patients with moderate CKD (CrCl of 20–50 mL/min) undergoing major orthopedic surgery, the relationship between kidney function and fondaparinux pharmacokinetics profiles was assessed [47]. Authors reported that fondaparinux trough and peak anti-Xa activity was lower in patients with CKD treated with 1.5 mg daily compared to patients with normal kidney treated with 2.5 mg daily (p < 0.01).
4.3.5 Clinical Practice
In the setting of CKD, UFH or potentially LMWHs are preferred over fondaparinux for anticoagulation therapy. However, because of its small molecule size, it does not have the same potential to cause heparin-induced thrombocytopenia (HIT) as UFH or LMWHs, and may be considered in patients with CKD when direct thrombin inhibitors (DTIs) or warfarin therapy are not feasible choices [36]. A case study in a dialysis patient with HIT showed that a fondaparinux dose of 2.5 mg every other day was a safe alternative anticoagulant [14].
5 Parenteral Direct Thrombin Inhibitors
In the settings of HIT or AT deficiency the choice of an optimal anticoagulant can be complicated and DITs can be a proper option for anticoagulation [36]. The most commonly DTIs used are argatroban, bivalirudin, and lepirudin. Unfortunately, pharmacotherapy trials for the DTIs did not consider the effect of kidney dysfunction, with the current dosing suggestions mostly influenced by postmarketing experiences [16].
Argatroban is hepatically excreted, and it has been proposed that no dose adjustment is necessary for CKD or hemodialysis. In trials, the average dose used in HIT therapy was 1.6 μg/kg/min, targeting aPTT values 1.5–3 times the baseline value [48]. Postmarketing studies of patients with moderate to severe CKD recommended a lower dosing strategy, with a dose reduction of approximately 0.1–0.6 μg/kg/min for each 30 mL/min decrease in the CrCl. In another study, the average argatroban dose to achieve target ranges was 0.8 μg/kg/min with a CrCl of below 30 mL/min, 1.2 μg/kg/min if CrCl was between 31 and 60 mL/min, and 2.2 μg/kg/min if CrCl was above 60 mL/min [48].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree