Receptor
P2Y1
P2Y12
Platelets, heart, blood vessels, smooth muscle cells, connective and neural tissues, testis
Platelets, some neural tissues, nasal mucosa, lymphocytes, endothelium
Gq
Gi2
Activation of PLC
Inhibition of AC, Activation of PI3K
Mobilization of calcium from intracellular stores
Platelet sustained aggregation
Platelet shape change
Potentiation of platelet secretion
Platelet transient aggregation
Impaired response to ADP
Greatly diminished aggregation in response to ADP
Absent increase in cytosolic Ca2+ and shape change in response to ADP
Absent inhibition of AC by ADP
Normal inhibition of cAMP formation by ADP
Increased bleeding time
A2P5P, A3P5P, MRS2179, MRS2279, MRS2500
AZD1283, MRS2395, AR-C69931MX, ticlopidine, clopidogrel, prasugrel, ticagrelor
Interactions between platelets and leukocytes mediated by platelet P-selectin exposure
Interactions between platelets and leukocytes mediated by platelet P-selectin exposure, Thrombin-induced exposure of PS, TF-induced thrombin formation
In this chapter, we focus on the molecular mechanisms and abnormalities of P2Y12 receptor in thrombosis aspects. Antiplatelet agents and therapies targeted on P2Y12 receptor are illustrated according to the latest clinical trial or animal experiments. Other roles of P2Y12 receptor in platelet apoptosis and inflammation are mentioned to give readers a comprehensive view.
2 Molecular Mechanisms and Effects of P2Y12 Receptor Signaling
2.1 Biochemistry Structure
P2Y12, as a G protein-coupled receptor, has the typical features of 7 hydrophobic transmembrane (TM) regions connected by 3 extracellular loops (EL) and 3 intracellular loops [14]. Human P2Y12 receptor consists of 342 amino acid residues [30]. Like most G protein-coupled receptors, P2Y12 receptor also possesses 4 extracellular cysteine residues at extracellular N-terminus (Cys17), 1st extracellular loop (Cys97), 2nd extracellular loop (Cys175), and 3rd extracellular loop (Cys270) [6]. Unlike P2Y1 receptor, which forms 2 disulfide bridges among the 4 extracellular cysteines essential for ADP-induced P2Y1 receptor activation [31], P2Y12 receptor extracellular cysteines Cys17 and Cys270 are not essential for ADP-induced P2Y12 receptor activation, and it seems that only one disulfide bridge is formed between Cys97 and Cys 175 [11, 32]. Thienopyridine antiplatelet drugs are believed to exert their antiplatelet roles by targeting Cys17 and Cys270 through their thiol group-containing active metabolites [32–34].
2.2 Agonists and Antagonists
ADP, released by ruptured red blood cells or platelets, is the natural agonist of P2Y12 and P2Y1 receptor [14]. The ADP analog 2-methylthio-ADP (2-MeSADP), frequently used in research, is a more potent and stable P2Y12 and P2Y1 agonist [11, 14, 25, 35].
Selective P2Y12 receptor antagonists include MRS1283 [23], MRS2395 [36], AR-C69931MX (Cangrelor) [37–39], nucleotide analog AZD6140 (Ticagrelor) [40–42]. and active metabolites of the thienopyridine compounds (Ticlopidine, Clopidogrel, Prasugrel) [14, 34, 43]. ATP and its triphosphate analogs like 2MeSATP and 2ClATP are selective P2Y12 receptor antagonists [44].
2.3 Signal Transductions
The network of P2Y12 receptor signal transduction is complicated and contradictory reports exist. Here, we categorize the pathways by basic G protein subunits and emphasize commonly reported functions of each pathway mentioned, focusing on the importance of P2Y12 receptor in multiple platelet functions.
2.3.1 Major Pathways
The P2Y12 receptor couples to Gα i2 subunit [6]. Upon stimulation, Gα and Gβγ subunits dissociate to activate various signal transduction pathways [12].
- (a)
Gαi2 subunit inhibits production of adenyl cyclase (AC), resulting in decrease of cAMP levels [45], which reduces the activation of cAMP-dependent protein kinase (PKA) [6]. PKA has a wide range of substrates in human platelets, including actin binding protein, caldesmon, Gα13, GPIbβ, IP3 receptors, phosphodiesterase 3, vasodilator-stimulated phosphoprotein (VASP), which all play important role in platelet functions [46].
- (b)
Gβγ subunit stimulates phosphatidylinositol-3 kinase (PI3K) activity, which results in late accumulation of phosphatidylinositol 3,4-bisphosphate [PtdIns(3,4)P2] and rapid transient accumulation of phosphatidylinositol 3,4,5-triphosphate [PtdIns(3,4,5)P3] [47–49]. PI3K pathway also activates Rap1b [13] and Akt [50]. Gβγ dimers can activate the G-protein-gated inwardly rectifying potassium channels (GIRKs) mediating Src tyrosine kinases [51].
2.3.2 Pathways in Platelet Activation
Platelet activation is a complex process in thrombosis and hemostasis induced by a variety of stimuli such as ADP, thrombin, collagen and thromboxane A2 (TxA2), which act cooperatively to ensure the rapid formation of a platelet thrombus at sites of vascular injury [45, 52]. The process mainly contains platelet shape change, adhesion, aggregation and secretion.
Platelet Aggregation
Platelet aggregation requires engagement of integrin αIIbβ3 by soluble fibrinogen [53]. The process starts by agonists that stimulate calcium release within platelets to activate the integrin αIIbβ3 on the platelet surface to bind soluble fibrinogen [13]. The activation of Gi-coupled receptors provides another independent signal to achieve full activation of platelet and stable aggregates [13]. P2Y12 receptor, connected to Gi2, participates in the process through following downstream signal pathways to activate integrin αIIbβ3.
- (a)
Inhibition of adenyl cyclase. Upon phosphorylation by PKA, actin binding protein and caldesmon may stabilize cytoskeleton of the resting platelets; IP3 receptors may down regulate the release of calcium from intracellular platelet stores; GPIbβ may prevent collagen-induced actin polymerization; and so on [46]. The decrease of cAMP level blocks the phosphorylation, so platelets tend to activate and aggregate. PKA is also responsible for inhibiting VASP by phosphorylation, which is an actin cytoskeleton regulatory protein that inhibits the integrin αIIbβ3 activation [54].
- (b)
Activation of PI3K. PI3K is directly activated by Gβγ subunit. In murine blood, absence of PI3Kγ led to formation of unstable thrombi, resulting in dissociation of multi-platelet aggregates; in addition, inhibiting PI3Kβ delayed initial thrombus formation and decreased individual platelet-platelet contact [55]. Persistent signaling from P2Y12 receptor to PI3Kβ and PI3Kγ isoforms is needed to sustain αIIbβ3 activation and maintain platelet aggregates [55].
- (c)
- (d)
Phosphorylation of Akt. It could be dependent on signaling through the P2Y12 receptor – PI3K pathway to activate integrin αIIbβ3 [50].
- (e)
Activation of ERK. A coordinated pathway through both Gq from TxA2 and Gi from ADP was necessary for activation of ERK2, involving in collagen-induced platelet aggregation and secretion [57, 58]. Activation of PLC and subsequent intracellular calcium increases occurring downstream of P2Y1 and Src activation occurring downstream of the P2Y12 receptor activation are both necessary for ADP-induced ERK2 activation [59]. PI3Kβ, mediating ADP-induced TxA2 generation by regulating ERK phosphorylation, also plays an important role in platelet aggregation [60].
- (f)
Activation of GIRK channels. Gβγ subunit activates the GIRK channels by binding to their cytosolic regions [51]. Co-stimulation of P2Y12 and P2Y1 receptors, through activation of both GIRK channels and Src family of tyrosine kinases, is essential for ADP-induced cPLA2 phosphorylation and TxA2 generation [51].
- (g)
Interaction with PAR. Protease activated receptor (PAR) 1 and PAR4 are thrombin receptors that have differential roles in platelet activation [61]. Thrombin activates the rapamycin complex-1 (mTORC1) pathway in human platelets through PAR-activated PKC-mediated ADP secretion and subsequent activation of P2Y12, in a manner largely independent of the canonical PI3K/Akt pathway [62]. PAR4, a low-affinity thrombin receptor in human platelets, participates in sustained platelet activation in a P2Y12-dependent manner [63]. Using bioluminescent resonance energy transfer technology, Khan et al. found that PAR4 and P2Y12 directly interacted to regulate Akt signaling after PAR4 activation in human embryonic kidney 293 T cells coexpressing PAR4 and P2Y12 receptors [61]. PAR4-P2Y12 association supports arrestin-mediated sustained signaling to Akt to stabilize platelet thrombi [61].
Platelet Shape Change
Platelet shape change (PSC) is an initiating process of platelet activation that leads to platelet aggregation. The P2Y1 receptor plays the major role in PSC but the P2Y12 receptor appears to be involved in ADP-induced PSC since this process was significantly inhibited by AR-C69931MX [18]. Research shows that P2Y12 receptor plays a potential role in ADP-induced PSC through regulation of the Rho kinase pathway, potentiating both myosin phosphorylation and actin polymerization [64].
Platelet Granule Release
Research shows that ADP-induced α granule release in aspirin-treated platelets occurs through co-stimulation of Gαq and Gαi signaling pathways and P2Y12 receptor plays an important role in TxA2-mediated α granule release [65]. Phosphorylation of Ephrin-receptor family members is mediated by P2Y1 and P2Y12 receptors, among which EphA4 is an intermediate in P2Y12 signaling to secretion thereby facilitating later stages of secondary aggregation and thrombus growth [19].
Thrombus Growth and Stability
Animal experiments demonstrate that ADP and its P2Y12 receptor participates in thrombus growth, especially in the formation of downstream part of the emboli from the initial thrombus [66]. This may explain the beneficial effects of P2Y12 receptor antagonists in secondary prevention of ischemic events in patients with arterial thrombosis [66]. Experiments on P2Y12-null mice demonstrated that P2Y12 receptor is involved in thrombus growth and stability [17].
2.3.3 Pathways in Platelet Apoptosis
Platelet apoptosis is the physiological way of platelet death if not forming thrombus. It is reported that the activation of P2Y12 receptor could protect platelets from apoptosis. Relative signaling pathways are hypothesized that the activation of P2Y12 receptor induces Akt and Bad phosphorylation and the phosphorylated Bad is sequestered in the cytoplasm by the adapter protein 14-3-3, which prevents a Bad association with Bcl-xL; therefore, free Bcl-xL heterodimerizes with Bak proteins to prevent Bak dimerization in mitochondria, thus antagonizing its proapoptotic activity [39, 67]. In an indirect way, Bcl-xL inactivates Bax by inhibiting its translocation into mitochondria [39]. From the view of preventing platelet apoptosis, we can see that the platelet lifespan could be shortened if P2Y12 receptor is antagonized, thus reducing thrombosis.
2.3.4 Pathways in Inflammation and Immunity
In recent decades, the role of platelets in inflammation emerges as a new research hotspot [68]. Taking thrombosis events in atherosclerosis as an example, platelets initiate the thrombus formation if the atherosclerotic plaque is ruptured and large numbers of inflammation factors are released from the ruptured site to the circulating blood [69]. Most studies demonstrate that platelets function as proinflammatory cells through granular release of inflammatory mediators or cytokines, and platelet-leukocyte interaction [70, 71]. In myocardial infarction mouse model, clopidogrel inhibited P-selectin expression, platelet-leukocyte aggregation and myocardial inflammation [69]. Therefore, P2Y12 receptor may contribute to cardiac inflammation after myocardial infarction.
Other disease models also demonstrate the importance of P2Y12 receptor in inflammation, but pro- or anti-inflammation varies in different studies. Paruchuri et al. found that leukotriene E4-induced murine pulmonary inflammation is mediated by P2Y12 receptor, suggesting that P2Y12 receptor may be a potential therapeutic target for asthma [72]. Platelet microparticles were identified from joint fluid of patients with rheumatoid arthritis or other forms of inflammatory arthritis, and were proved proinflammatory for stimulating cytokine responses from synovial fibroblasts through IL-1 [73]. However, using rat model of peptidoglycan polysaccharide (PG-PS)-induced arthritis, Garcia et al. found that clopidogrel, the widely-used antiplatelet drug targeting P2Y12 receptor, exaggerated the inflammatory response [74], supporting the anti-inflammatory role of P2Y12 receptor in arthritis. Consistently, using an LPS-induced systemic inflammation mouse model, the same group shows that P2Y12 knockout aggravates inflammatory injury, showing the protective role of P2Y12 receptor against inflammatory injury [75]. In contrast, in a LPS-induced systemic inflammation rat model, clopidogrel pretreatment reduces inflammatory damage of lung and liver [76]. The discrepancy cannot be simply attributed to species difference, because the protective effect of clopidogrel was also observed in a mouse model of polymicrobial sepsis [77]. Further work is needed to elucidate the causal relation between antiplatelet effects of clopidogrel and inflammation [78]. A simplified overview of P2Y12 receptor signaling pathways and its co-transduction pathways with P2Y1 receptor and PAR receptor is illustrated by Fig. 1.
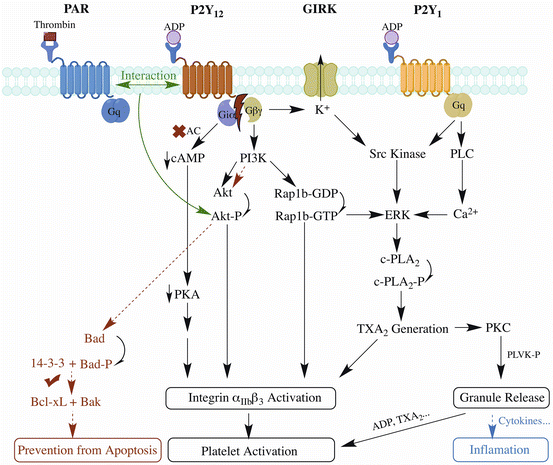

Fig. 1
A simplified overview of P2Y12 receptor signaling pathways and its co-transduction pathways with P2Y1 receptor and PAR receptor [39, 79]. P2Y12 and P2Y1 receptors are activated by ADP while PAR receptor by thrombin. Once P2Y12 activated, Giα inhibits adenyl cyclase to reduce cAMP level; Giβγ activates PI3K and GIRK to induce downstream activation of Akt, Rap1b and Src kinase. P2Y1 receptor costimulates Src kinase or ERK through Gq signaling. PAR receptor costimulates Akt by interaction with P2Y12 receptor. Effects include αIIbβ3 integrin activation, resulting in platelet activation and aggregates stabilization. Generation of TxA2 induces both integrin activation and granule release, in which small molecules like ADP continues to activate platelets and cytokines may induce inflammation. The anti-apoptotic pathway of P2Y12 receptor is mainly through Akt pathway to phosphorylate Bad to enable the binding of Bcl-xL with Bak thus achieving antiapoptotic effects
3 Abnormalities of P2Y12 Receptors
3.1 P2Y12 Gene Polymorphisms
There are four P2Y12 gene polymorphisms in total linkage disequilibrium, determining haplotypes H1 and H2, with respective allelic frequencies of 0.86 and 0.14 [80]. Carriers of the H2 haplotype exhibit increased ADP-induced platelet aggregation; thus they may have an increased risk of atherothrombosis or a lesser clinical response to drugs inhibiting platelet function [80]. Several studies show that H2 haplotype is associated with the risk of peripheral arterial disease (PAD) or contributes to clopidogrel resistance [81, 82]. However, most studies report that P2Y12 polymorphisms are not associated with platelet-relative diseases such as coronary artery disease (CAD) or altered platelet function inhibition by P2Y12 antagonists [83–89]. Whether people of H2 haplotype have a tendency of thrombotic events are not clearly evidenced yet.
3.2 Congenital Deficiency of P2Y12 Receptor
Congenital P2Y12 deficiency is an autosomal recessive disorder [90]. Patients of congenital severe P2Y12 deficiency exhibit excessive bleeding and prolonged bleeding time [91]. Coagulant defects in patients of heterozygous P2Y12 deficiency are less severe, mainly characterized by that low concentrations of ADP (≤10 μM) can only induce reversible platelet aggregation [92]. Treatment for these patients is intravenous infusion of desmopressin, a vasopressin analog [93].
3.3 P2Y12 Receptor Abnormal Expression in Diseases
P2Y12 receptor abnormal expression is detected in some diseases, which may explain the high rate of clinical phenomenon “clopidogrel resistance” (referring to failure of clopidogrel to achieve antiplatelet effects) in some patients of certain diseases [54]. Typical diseases are listed below for references but further elucidation of the molecular mechanism is needed.
3.3.1 Diabetes Mellitus
Platelet function in diabetes mellitus patients is altered in the following aspects: accelerated platelet turnover, increased TXA2 release and platelet aggregation [94, 95]. Diabetes patients, with standard clopidogrel treatment or combined aspirin and clopidogrel treatment, have high on treatment platelet reactivity (HTPR) [96], an independent risk factor of recurrent ischemic events [97]. It is hypothesized that P2Y12 receptor expression is upregulated or the downstream signaling is amplified in diabetes patients [98–100]. We found that type II diabetes mellitus patients have increased P2Y12 expression on platelet, and the platelet P2Y12 level correlates with platelet reactivity to multiple agonists including ADP, thrombin and AYPGKF (Hu et al., unpublished data). For diabetes patients with “clopidogrel resistance”, alternative antiplatelet drugs like prasugrel or ticagrelor can be used for preventing cardiovascular events [101]; adjunctive use of cilostazol can also be applied to reduce platelet reactivity in diabetes patients [102].
3.3.2 Chronic Kidney Disease
Like diabetes, chronic kidney disease patients have high residual platelet reactivity (HRPR); chronic kidney disease and diabetes confer a synergistic impact on HRPR [103]. Studies from randomized trials suggest that renal function has an influence on clinical efficacy of clopidogrel [104]. Whether P2Y12 receptor upregulation or increased signaling activation downstream of P2Y12 participates in the increased platelet reactivity and impaired clopidogrel response in chronic kidney disease, as in the case of diabetes, is not clear.
4 Antiplatelet Drugs Targeting P2Y12 Receptor
The common pathological basis of coronary artery disease and ischaemic stroke is the arterial thrombosis, a result of aberrant platelet activation. P2Y12 plays a central role in platelet activation [105] and mainly distributed in platelets [6], therefore P2Y12 is an ideal target for antiplatelet drug development. In fact, P2Y12 receptor is the most successful antiplatelet target so far. Four P2Y12 receptor antagonists have been approved by FDA as antiplatelet drugs, including ticlopidine, clopidogrel, prasugrel, and ticagrelor. Among them, ticlopidine, clopidogrel and prasugrel belong to thienopyridine and are prodrugs, which need to be transformed in liver to form active metabolite to exert their P2Y12 antagonizing roles [106].
Clopidogrel is the most widely used antiplatelet drug antagonizing P2Y12 receptor with proved benefits over aspirin and the first P2Y12 antagonist ticlopidine. The main limitations of clopidogrel include slow onset, slow offset of action, modest platelet inhibition, individual variability compared with 3rd generation of thienopyridine P2Y12 receptor antagonist prasugrel and direct P2Y12 receptor antagonist ticagrelor [43, 79]. Besides, P2Y12 receptor inverse agonists exhibit more potent antiplatelet effects on platelets expressing constitutively active P2Y12 receptor, and therefore may have better antithrombotic efficacy [35, 37]. Dual antiplatelet therapy (DAPT) has been the standard of care for patients with ACS and those undergoing stenting, which triggers researches on antiplatelet compounds with dual or multiple targets like BF061 [107]. In this part, we will briefly introduce the antiplatelet agents targeting P2Y12 receptor in clinical use and under development. For more detailed information about the pharmacology and clinical use of the marketed P2Y12 receptor antagonists, please refer to two excellent reviews published recently [43, 108].
4.1 Thienopyridines
Among the five marketed P2Y12 receptor antagonists, ticlopidine, clopidogrel and prasugrel belong to thienopyridine family. All of them are prodrugs, which are transformed into thiol-containing active metabolites and covalently bind to P2Y12 receptor [106]. The binding inhibits ADP-induced platelet activation irreversibly.
4.1.1 Ticlopidine
Ticlopidine is the first antiplatelet drug targeting P2Y12 receptor in thienopyridine family.
Pharmacodynamics and Pharmacokinetics
Side Effect
Ticlopidine has severe side effects including neutropenia, aplastic anemia, thrombotic thrombocytopenic purpura (TTP) and gastrointestinal reactions, which limit its clinical uses [111].
Clinical Application
Ticlopidine is seldom used in acute ischemic cardiovascular events due to its slow onset and the serious adverse effects described above. Studies showed that high administration dose (500 mg daily) with aspirin could have rapid antiplatelet effects in ACS patients [112].
4.1.2 Clopidogrel
Clopidogrel is the second generation of thienopyridines that outweighs ticlopidine with better effects against platelet aggregation and fewer side effects [113]. In 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes, clopidogrel is under class I recommendation [114].
Pharmacodynamics and Pharmacokinetics
Eighty five to ninety percent of the absorptive drugs are hydrolyzed to inactive metabolites by esterase and only 10–15 % are converted into active metabolites by hepatic CYP [109]. The active metabolites bind irreversibly to the cysteine residue of P2Y12 extracellular domain and the effect could last the whole life span of platelets [106]. The onset and peak time are similar to ticlopidine and the metabolites also excrete from both urine and feces [109].
Clinical Application
Clopidogrel is the routine medicine in preventing thrombotic events in diseases like ACS, the loading dose administration of which could have apparent antiplatelet aggregation effects before ACS patients undergo percutaneous coronary intervention (PCI) [115]. For patients with ST-segment elevation myocardial infarction (STEMI), an ACS clinical manifestation of high mortality, if clopidogrel was used as an adjunctive therapy to support reperfusion with primary PCI, the loading dose is 600 mg as early as possible or at the time of PCI, 75 mg daily thereafter; if with fibrinolytic therapy, loading dose is 300 mg by 75 mg daily [116]. Some studies also show that a high loading dose of 900 mg could give stronger inhibition of platelet aggregation but the safety issue is questionable [117].
Side Effects
Clopidogrel could induce severe rashes in some patients and gastrointestinal reactions such as nausea and vomiting, with rare cases of neutropenia compared with ticlopidine [118]. Clopidogrel is also a cause of gastrointestinal bleeding [119]. But in the case of co-medication of clopidogrel and proton pump inhibitors (PPIs), PPIs with weaker inhibition of CYP2C19 are preferred due to its potential negative clinical impacts on therapeutic efficacy of clopidogrel [120]. Although clopidogrel is widely used clinically, it’s still not an ideal antiplatelet drug. Low onset is mainly due to the metabolism process in the liver [106]. Individual variability is associated with genes related to drug metabolism, which may cause “clopidogrel resistance” and impair the prevention from thrombotic events among certain patient groups [54, 121–123]. Besides the gene polymorphism that causes change of CYP activity, “clopidogrel resistance” could also result from increased platelet P2Y12 in some patients such as in type II diabetes mellitus [37] and the low inverse agonist activity (Hu et al., unpublished data), or combination of multiple factors [124]. Moreover, clopidogrel, as an irreversible P2Y12 receptor antagonist, could retard the recovery of platelets after withdraw; thus clopidogrel has the bleeding risk if emergency surgery is needed.
4.1.3 Prasugrel
Prasugrel (CS-747, LY640315) is the third generation of thienopyridines.
Pharmacodynamics and Pharmacokinetics
Clinical Application
The phase III clinical trial of prasugrel (a 60 mg loading dose and a 10 mg daily maintenance dose) compared with clopidogrel (a 300 mg loading dose and a 75 mg maintenance dose) showed that prasugrel therapy owned significantly reduced rates of ischemic events in patients with ACS treated by PCI, but had an increased risk of major bleeding events including fatal bleeding; consequences were that mortality rate was similar between those two groups [127]. Conclusions still differ in many recent researches investigating whether prasugrel has more advantages over clopidogrel for ACS: Delia et al. did retrospective investigation of 525 ACS in-hospital patients and the results showed no significant changes, after changing clopidogrel to prasugrel, in terms of bleeding or thrombotic events [128]. Olson et al. compared 10,963 ASC patients taking clopidogrel or prasugrel in Truven Health Analytics MarketScan database, and concluded that the two had similar effects while clopidogrel is better than prasugrel in the long run [129]. In contrast, Koziński et al. demonstrated that prasugrel could lower the high on-treatment platelet reactivity (HTPR) in 71 ACS patients who showed HTPR after taking clopidogrel [130]. So prasugrel is still not able to substitute clopidogrel as routine medication for ACS patients; it is an alternative for patients who have “clopidogrel resistance”. The reason behind may be that mutations of CYP2C9 and CYP2C19 affect platelet aggregation heavily on clopidogrel but not on prasugrel [131].
Side Effects
Clinical trials revealed excess bleeding mainly occurring in maintenance phase, which reminds that patient selection for prasugrel is necessary [126].
4.2 P2Y12 Receptor Direct Antagonists
In order to reach rapid and reversible antiplatelet effects, P2Y12 receptor direct antagonists with short half life are developed.
4.2.1 Ticagrelor
Ticagrelor (AZD6140, Brilinta) is the first marketed P2Y12 receptor antagonist that reversibly and directly blocks P2Y12 receptor; it is a cyclopentyltriazolopyrimidine and can be used orally [132, 133]. Radioligand binding assay demonstrates that ticagrelor is a non-competitive antagonist that has a binding site in P2Y12 receptor independent of ADP [42, 133].
Pharmacodynamics and Pharmacokinetics
Compared to thienopyridines, ticagrelor can inhibit P2Y12 receptor directly without any hepatic biological conversion [133]. The inhibition rate could reach 95 % in 2–4 h and it can take effects in 2 h without loading dose [132]. The half life is 6–9 h so that residual effect is shorter than thienopyridines [134]. Tests of [14C]-labeled ticagrelor administrated in healthy subjects showed that the mean radioactivity recovery was 58 % from feces and 27 % from urine [135]. Gene polymorphism of CYP has no impacts on ticagrelor so it is effective for patients with “clopidogrel resistance” [136].
Clinical Application
For ACS patients with or without ST-segment elevation, ticagrelor of 180 mg loading dose and 90 mg twice daily thereafter could significantly reduce the cardiovascular events compared with clopidogrel of 300–600 loading dose and 75 mg daily thereafter [137]. Ticagrelor has higher and more consistent antiplatelet effects and could lower mortality of cardiovascular diseases, myocardial infarction and stroke in ACS patients and patients with prior myocardial infarction [138–140]. Shah et al. ever reported that ticagrelor can be used as alternative in clopidogrel-induced neutropenia [141].
Side Effects
In a phase III clinical trial, ticagrelor was demonstrated to increase non-procedure related bleeding in 18,624 ACS patients with or without ST-segment elevation [137]. Dyspnea was more frequently observed in patients using ticagrelor than clopidogrel, which might be due to the constant inhibition of P2Y12 receptors on neurons by ticagrelor resulting in increased sensitivity to dyspnea [142].
4.2.2 Cangrelor
Cangrelor (ARC69931MX) is a direct and reversible P2Y12 antagonist. It is developed as antiplatelet agent for intravenous use with rapid onset and offset action. Structurally, cangrelor is the analog of ATP, the weak endogenous antagonist of P2Y12 receptor [143]. Cangrelor has won European approval in March 2015 and may also get approved by FDA as an intravenous antiplatelet drug to prevent thrombosis during angioplasty.
Pharmacodynamics and Pharmacokinetics
Clinical Application
Cangrelor is administrated intravenously [144]. Champion-Phoenix in more than 11,000 patients undergoing PCI, demonstrated that cangrelor is superior to clopidogrel to reduce the combined risk of death, heart attack, repeat procedures and stent thrombosis [145]. Compared with clopidogrel, cangrelor slightly increased bleeding.
4.2.3 Elinogrel
Elinogrel (PRT060128), is a potent competitive direct P2Y12 receptor antagonist developed for both oral and intravenous use [146, 147]. The development of elinogrel was terminated before phase III study by Novartis in 2012.
Pharmacodynamics and Pharmacokinetics
The average half life is 11 h of 40 mg elinogrel administered intravenously, and the peak antiplatelet effect reaches in 20 min but totally reversed in 8–24 h [148].
Clinical Application
Elinogrel can be orally or intravenously administered, in two pharmacologically identical forms with different dosages [147]. In phase I clinical trial, elinogrel had potent antiplatelet aggregation effects with similar bleeding side effects as the placebo group [149]. In phase II clinical trial for non-urgent patients undergoing PCI, the potential bleeding risk of elinogrel was higher than clopidogrel [150].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree