Absolute contraindications:
*Active internal bleeding or DIC
*Recent cerebrovascular event (including TIA)
*Recent neurosurgery or intracranial trauma (<3 months)
*Absolute contraindication to anticoagulation
Relative contraindications:
*Recent CPR, major surgery, obstetrical delivery (< 7–10 days)
*Recent organ biopsy, major trauma, or cataract surgery (<7–10 days)
*Intracranial tumor, other intracranial lesion, or seizure disorder
*Recent major GIS bleeding or internal eye surgery (<3 months)
*Serious allergic reaction to thrombolytic agent, anticoagulant or contrast media
*Known right-to-left cardiac or pulmonary shunt, left heart thrombus
*Severe dyspnea or severe acute medical illness precluding safe procedure
*Suspicion for infected venous thrombus
*Renal failure (GFR < 60 mL/min)
*Short life expectancy
*Severe thrombocytopenia
*Uncontrolled hypertension
*Bacterial endocarditis
*Pregnancy or lactation
*Severe hepatic dysfunction
*Diabetic hemorrhagic retinopathy
1.1 Endovascular Interventional Options for Deep Vein Thrombosis
1.1.1 Catheter-Directed Thrombolysis (CDT) for DVT
Image-guided, catheter-directed, intra-thrombus drug delivery has been developed for improving the safety and efficacy of thrombolytic therapy for thromboembolic disease. CDT has several advantages for DVT patients and can be used:
- 1.
To achieve high intra-thrombus thrombolytic agent concentrations.
- 2.
To avoid bypass of the drug via collaterals around the thrombosed vein.
- 3.
To reduce thrombolytic agent dose, treatment time, intensive care utilization and hospitalization time.
- 4.
To decrease bleeding complications.
- 5.
To treat underlying venous abnormalities by other endovascular techniques.
Catheter-directed thrombolysis (CDT) means delivery of a thrombolytic drug (rtPa, Urokinase) directly into the thrombus using a catheter or catheter-like device that is embedded within the thrombus by using Doppler ultrasound and fluoroscopy guidance. Usually a standard multisidehole catheter might be used but recently a multisidehole catheter that simultaneously applies ultrasound energy (EkoSoniccatheter; EKOS) to improve drug delivery into the thrombus has also been developed and this device is expected to increase safety and efficacy [4, 5, 10] (Fig. 1).
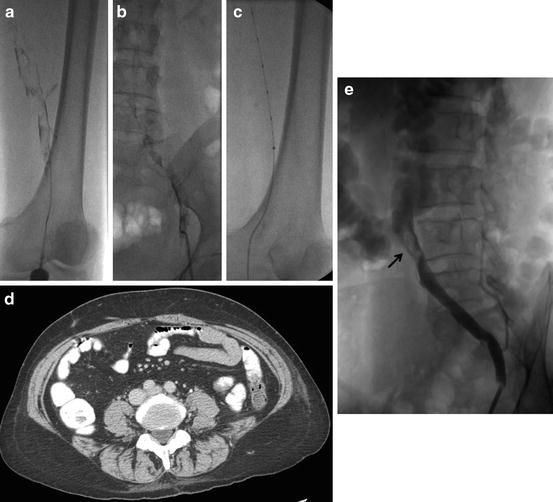

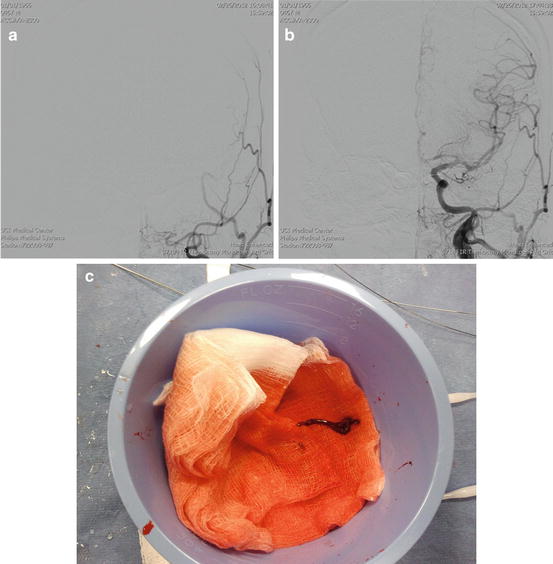
Fig. 1
Left iliofemoral DVT (a, b), placement of ultrasonic EKOS catheter with improved drug delivery into the thrombus (c), CT examination was obtained because of the suspicion of May-Thurner syndrome but the compression on left common iliac vein was moderate (d), only a small partial thrombus (arrow) was visible after the treatment (e)
Currently, the most commonly used fibrinolytic drug for DVT is the recombinant tissue plasminogen activator (rtPA). The drug is infused continuously and directly into the thrombus at a low dose (0.5–1.0 mg/h) with systemic intravenous infusion of unfractionated heparin at subtherapeutic levels. Mostly the patient is closely monitored in an intensive care unit. Infusion might be stopped in case of active bleeding or severely abnormal coagulation parameters. Patients are evaluated by venography 1–2 times a day until the patency is achieved. Venous balloon angioplasty with thrombolytic infusion may also be used after partial thrombolysis [4, 5]. Left iliac anatomical venous stenosis (May-Thurner Syndrome) is usually treated by venous stent implantation and other venous stenoses might also be treated by balloon angioplasty and/or stent placement [4, 13]. Venography is performed to confirm the patency of the venous system. Before the patient is discharged anticoagulant therapy is arranged and compression stockings are prescribed. CDT has been evaluated in many case series and prospective multicenter trials. Major bleeding was observed in 5–12 % of patients in some older studies. However, more recent series with RCT reported much lower major bleeding rates [4, 5, 11, 12]. Fatal PE after CDT has also been reported very rarely [4, 14]. Although CDT is effective, currently the technique is not used widely because of long infusion times with intensive monitoring that are associated with longer hospital stays, and the risk of systemic bleeding still exists.
1.1.2 Pharmacomechanical Catheter-Directed Thrombolysis
Pharmacomechanical catheter-directed thrombolysis (PCDT) includes intrathrombus thrombolytic agent infusion with mechanical thrombectomy devices to improve drug penetration into thrombus and macerate thrombus for aspiration or percutaneous thrombectomy [5, 11]. These devices enable faster penetration of thrombolytic agent within the thrombus, accelerating successful thrombolysis and improving safety by reducing drug dose and exposure time. Successful use of PCDT has been described in a number of published DVT studies [15–17]. Recently, new PCDT devices have been introduced that can enable endovascular DVT therapy to be completed in a single procedure session without the need for further drug infusions or intensive care monitoring. AngioJet Thrombectomy System (Boston Scientific) gives forceful pulse-spray bolus dose of the thrombolytic drug directly into the thrombus [18]. The drug is allowed to interact within the thrombus for a while and the device is used to aspirate the residual thrombus at the end. Isolated thrombolysis is another method performed by Trellis Peripheral Infusion System (Covidien) [5, 19]. With this device two catheter-mounted balloons are inflated to isolate a segment of vein and a bolus dose of a thrombolytic drug is injected directly into the thrombus. Activation of an oscillating wire for 10 min is then used to mechanically disperse the drug within the thrombus, and then the drug and liquefied debris are aspirated through a port on the device. Another similar device is Reya Thrombectomy catheter (Biolas Health) that is designed to use with the implantation of a temporary retrievable vena cava filter for protection before activation of an oscillating wire (Fig. 2). There are also many different endovascular venous thrombus aspiration systems such as Aspirex S Catheter (Straub Medical) and Angiovac Cannula and Circuit (AngioDynamics).
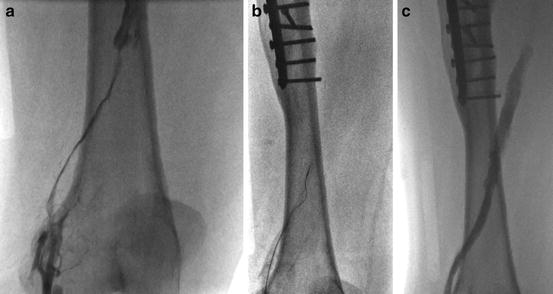

Fig. 2
Right iliofemoral DVT, thrombus is occlusive at the popliteal segment (a), oscillating wire activation of the pharmacomechanical thrombectomy device (b), recanalization of the thrombosed segment within minutes (c)
1.1.3 Percutaneous Mechanical Thrombectomy
Stand-alone percutaneous mechanical thrombectomy (PMT) refers to the percutaneous use of catheter-based mechanical devices that contribute to thrombus removal via fragmentation, maceration, and/or aspiration, without administration of a thrombolytic drug. These methods are not always suitable for every patient and in most of the cases they are reserved for the patients with serious risk of bleeding and/or other contraindications for the thrombolytic therapy. PMT has not received general acceptance because of the associated risk of PE and damage to venous valves caused by thrombectomy devices [10, 13].
1.1.4 Percutaneous Aspiration Thrombectomy
Percutaneous aspiration thrombectomy (PAT) technique can be defined as using a syringe to aspirate thrombus from the vein via a catheter, device, or sheath. PAT has been routinely used to effectively eliminate thrombi located in hemodialysis fistulae with a patency rate of 86 % at 6 months after PAT [21]. PAT also has been accepted as a rapid, safe, and effective method of management of iliofemoral vein thrombosis and provides higher recanalization rates than alternative treatments [13]. However, few clinical studies have been performed. Previous studies reported a recanalization rate of 88.9 % with PAT for the lower extremity DVT. Mechanical thrombectomy devices are not required during PAT and, therefore, the risk of trauma to the vascular wall and valves is thought to be low. Thrombolytic agents are not given to PAT patients, and the bleeding complications associated with systemic therapy are therefore absent [13, 22]. However there is no standardized PAT aspiration method and the technique is not widely accepted. PAT is most commonly used as an adjunctive endovascular technique like balloon maceration to fragment thrombus, balloon angioplasty, stent implantation and vena cava filter placement (Fig. 3).
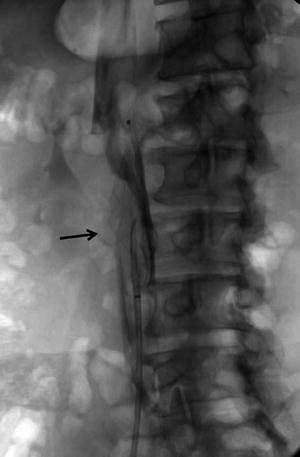

Fig. 3
Retrievable IVC filter was placed for periprocedural PE prophylaxis during pharmacomechanical thrombolytic treatment. Significant thrombus material (arrow) was captured by the filter just after the treatment
Interventional endovascular therapies for DVT have the potential to provide PE protection and prevention of PTS. There is increasing number of scientific evidence in support of endovascular treatments. However, patient centered individualized approach for endovascular DVT treatment is recommended to optimize the ideal clinical result.
2 Endovascular Treatment of Acute Stroke
According to the World Health Organization (WHO), stroke is the leading cause of death for people above the age of 60 and the fifth leading cause in people aged 15–59 [23]. Stroke is the most common cause of disability worldwide [23, 24]. Every 6 s someone in the world will either be permanently disabled or will die due to stroke. More than 80 % of stroke cases are acute ischemic stroke cases due to cessation or diminution of blood supply to a certain part of the brain after arterial occlusion or hypoperfusion. Acute ischemia occurs mostly due to thromboembolism or local occlusion. Less frequently global hypoperfusion may cause acute cerebral ischemia. Immediate and prompt treatment of acute stroke is very important because of the heavy burden of this disease on a person and the society. Mortality during the first 30 days of ischemic stroke is 20 % and 30 % of survivors will remain permanently disabled [25].
2.1 Primary Management of Acute Stroke Patients
An acute stroke patient first seen in a hospital with the capability of intravenous (IV) fibrinolysis must have a nonenhanced computerized tomography (NECT) of the head to rule out cerebral hemorrhage. Patients without significant improvement after IV fibrinolysis can be immediately transferred to higher-level stroke centers. Patients that are seen by the emergency medical services in the field will benefit more if they can be sent to the nearest stroke center with the capability of both intravenous and intraarterial therapy and dedicated neurovascular imaging capabilities. IV recombinant tissue plasminogen activator (alteplase, rtPA) was the first approved treatment for acute ischemic stroke within 3 h of stroke onset after the NINDS (National Institute of Neurological Disorder and Stroke) trial. There are several exclusion criteria for the IV fibrinolytic therapy (Table 2).
Table 2
Inclusion and exclusion characteristics for patients with ischemic stroke who could be treated with IV rtPA within 3 h from symptom onset
Inclusion criteria |
Diagnosis of ischemic stroke causing measurable neurological deficit |
Onset of symptoms <3 h before beginning treatment |
Aged ≥18 years |
Exclusion criteria |
Significant head trauma or prior stroke in previous 3 months |
Symptoms suggest subarachnoid hemorrhage |
Arterial puncture at noncompressible site in previous 7 days |
History of previous intracranial hemorrhage |
Intracranial neoplasm, arteriovenous malformation, or aneurysm |
Recent intracranial or intraspinal surgery |
Elevated blood pressure (systolic >185 mm Hg or diastolic >110 mm Hg) |
Active internal bleeding |
Acute bleeding diathesis, including but not limited to |
Platelet count <100,000/mm |
Heparin received within 48 h, resulting in abnormally elevated aPTT (activated partial thromboplastin time) greater than the upper limit of normal |
Current use of anticoagulant with international normalized ratio (INR) >1.7 or partial thromboplastin time (PT) >15 s |
Current use of direct thrombin inhibitors or direct factor Xa inhibitors with |
elevated sensitive laboratory tests (such as aPTT, INR, platelet count), and |
ECT (ecarin clotting time); TT (thrombin time); or appropriate factor Xa activity assays |
Blood glucose concentration <50 mg/dL (2.7 mmol/L) |
CT demonstrates multilobar infarction (hypodensity >1/3 cerebral hemisphere) |
Relative exclusion criteria Recent experience suggests that under some circumstances—with careful consideration and weighting of risk to benefit—patients may receive fibrinolytic therapy despite 1 or more relative contraindications. Consider risk to benefit of IV rtPA administration carefully if any of these relative contraindications are present: |
Only minor or rapidly improving stroke symptoms (clearing spontaneously) |
Pregnancy |
Seizure at onset with postictal residual neurological impairments |
Major surgery or serious trauma within previous 14 days |
Recent gastrointestinal or urinary tract hemorrhage (within previous 21 days) |
Recent acute myocardial infarction (within previous 3 months) |
The American Stroke Association (ASA, 2013) published guidelines for the early management of patients with acute ischemic stroke [26] (Tables 3, 4 and 5).
Table 3
ASA recommendations for the evaluation of the stroke patient in the emergency service
1. An organized protocol for the emergency evaluation of patients with suspected stroke (Class 1; Level of evidence B). The goal is to complete and begin fibrinolytic treatment within 60 min of the patient’s arrival in an ED (Emergency department). Designation of an acute stroke team. Patients with stroke should have a careful clinical assessment, including neurological examination |
2. The use of stroke rating scale preferably the NIHSS (National Institute of Health Stroke Scale) (Class 1; Level of evidence B) |
3. A limited number of hematologic, coagulation and biochemistry tests are recommended during the initial emergency evaluation, and only the assessment of blood glucose must precede the initiation of intravenous rtPA (Class 1; Level of evidence B). Because hypoglycemia may mimic stroke signs and hyperglycemia is a risk factor for unfavorable outcomes |
Table 4
ASA recommendations for the radiologic evaluation of the stroke patient
1. Either head NECT (Nonenhanced computerized tomography) or MRI (Magnetic resonance imaging) is recommended before intravenous rtPA administration to exclude intracerebral hematoma which is an absolute contraindication (Class 1; Level of evidence A) |
2. Intravenous fibrinolytic therapy is recommended in the setting of early ischemic changes (other than frank hypodenisty) on CT, regardless of their extent (Class I; Level of evidence A) |
3. A noninvasive intracranial vascular study is strongly recommended during the initial imaging evaluation of the acute stroke patient if either intraarterial fibrinolysis or mechanical thrombectomy is contemplated for management but should not delay intravenous rtPA if indicated (Class I; Level of evidence A) |
4. CT perfusion and MRI perfusion and diffusion imaging, including measures of infarct core and penumbra, may be considered for the selection of patients for acute reperfusion therapy beyond the time windows for intravenous fibrinolysis. These techniques provide additional information that may improve diagnosis, mechanism, and severity of ischemic stroke and allow more informed clinical decision making (Class IIb; Level of Evidence B) |
5. Frank hypodensity on NECT may increase the risk of hemorrhage with fibrinolysis and should be considered in treatment decisions. If frank hypodensity involves more than one third of the middle cerebral artery (MCA) territory, intravenous rtPA treatment should be withheld (Class III; Level of Evidence A) |
Table 5
ASA recommendations for intravenous fibrinolysis
1. Intravenous rtPA (0.9 mg/kg, maximum dose 90 mg) is recommended for selected patients who may be treated within 3 h of onset of ischemic stroke (Class I; Level of Evidence A). Physicians should review the criteria outlined in Table 2 (which are modeled on those used in the NINDS Trial) to determine the eligibility of the patient |
2. Intravenous rtPA (0.9 mg/kg, maximum dose 90 mg) is recommended for administration to eligible patients who can be treated in the time period of 3–4.5 h after stroke onset (Class I; Level of Evidence B). The eligibility criteria for treatment in this time period are similar to those for people treated at earlier time periods within 3 h, with the following additional exclusion criteria: patients >80 years old, those taking oral anticoagulants regardless of INR, those with a baseline NIHSS score >25, those with imaging evidence of ischemic injury involving more than one third of the MCA territory, or those with a history of both stroke and diabetes mellitus |
Recommendations with the highest level of evidence and that are directly related with the endovascular management of acute stroke are included. Recommendations regarding the intensive care management of acute stroke patients are not within the scope of this article. According to these guidelines an algorithm for management of acute stroke patients can be formed (Table 6).
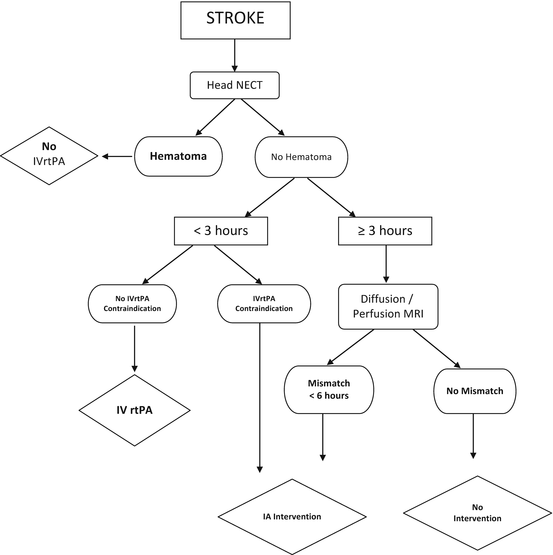
Table 6
Algorithm for management of acute stroke patients

2.2 Endovascular Therapy
Recanalization rates after IV rtPA in internal carotid artery terminus and MCA M1 segment occlusions range between 10 and 50 % and less than 40 % of patients regain functional independence [27]. Endovascular therapy has the main advantage of extended therapy window, earlier and more efficient recanalization and less risk of hemorrhage due to lower doses of thrombolytics. Endovascular therapies include either application of local intraarterial thrombolytics in the occluded intracranial segment or mechanical removal of the clot with a thrombectomy device or another endovascular device like a balloon or stent. According to most recent guidelines safe therapy window for endovascular treatment is the first 6 hours after the stroke onset [26]. After the development of dedicated mechanical thrombectomy devices, including stent retrievers, the balloon angioplasty has been put aside. Major disadvantages of endovascular therapies include low availability due to requirement of high level of expertise of specialists and complex neurointerventional infrastructure, delay in therapy initiation and invasive nature of the endovascular methods. Potential complications of endovascular stroke treatment are reperfusion hemorrhage, distal emboli, intracranial dissections, hematomas and subarachnoid hemorrhage [28, 29].
2.2.1 Intraarterial Fibrinolysis
The first positive randomized trial [28] to evaluate the efficiency of intraarterial fibrinolysis was PROACT 2 (Prolyse in Acute Cerebral Thromboembolism). In this study IA fibrinolysis with recombinant prourokinase (r-pro-UK) yielded higher middle cerebral artery recanalization rates than the control group (66 % vs % 18, p < 0.001). Fibrinolytics used in acute stroke treatment work as plasminogen activators. Prourokinase did not achieve FDA approval and it is not available any more and today the most frequently used IA fibrinolytic is Alteplase (rtPA). Alteplase is applied directly within the thrombus through a microcatheter. Most neurointerventional specialists would use a maximum dose of 22 mg of IA rtPA [30, 31]. Adjunct mechanical disruption of the clot can be used with microwire maneuvers to increase the interaction surface area of the clot and the medicine. Recanalization rates in large artery occlusion including internal carotid artery, basilar artery or MCA M1 segment is not as high as distal and smaller arteries [32, 33]. Due to this reason in most centers first line treatment for large artery occlusions causing stroke is the mechanical thrombectomy. However, IA fibrinolysis is still used very efficiently in distal small artery occlusions like MCA M2-M3 segments, ACA paricallosal/callosomarginal branches and posterior cerebral artery occlusions. Bridging therapies as combination of IV rtPA and IA rtPA can further increase recanalization rates. Three consecutive trials, interventional management of stroke (IMS) 1, 2 and 3, showed better outcomes with comparable rates of symptomatic intracranial hematoma and mortality compared with NINDS rtPA trial [30, 34, 35].