The United Kingdom has one of the highest incidences of breast cancer (123 per 100,000 women were diagnosed with breast cancer in 2005) and has historically been home to pioneers of breast cancer treatments.
Without being exhaustive, the trials originating in the United Kingdom over the last century have contributed significantly to improving our understanding about breast cancer and changed practice worldwide. Many of these trials have a story behind them that illustrates how conceptual leaps essential for progress in medicine are taken. The current TARGIT (TARGeted Intraoperative radioTherapy) trial is a story that will be highlighted because it demonstrates the interweaving of various new concepts in the evolution of local treatment of breast cancer.
Radical curative surgery for breast cancer was championed by William Halsted in North America and H. Sampson Handley and Gordon-Taylor in the United Kingdom and Europe. David Patey, working in the Middlesex Hospital in London, was one of the first to suggest in 1948 that removal of the pectoralis muscle was not necessary and that local treatment of breast cancer could be achieved without undue morbidity.1 Several trials were conducted in the latter half of the 20th century including many from the United Kingdom. The number of patients in these trials was small and the concept of statistical power was ill understood. Consequently, although these trials of local therapy demonstrated how the benefit of radiotherapy was a clear reduction in local recurrence rate by two-thirds, most of these trials did not show any survival advantage, thus apparently supporting the Fisher hypothesis that local control does not in any way influence distant disease. This concept was challenged only because of 2 events—the amalgamation of all trial data so the number of patients was adequate, and the longer follow-up that was almost necessitated by the continuing presence of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). It was the efforts of EBCTCG’s review of randomized previously performed trials that has now conclusively demonstrated that local control influences distant disease, although the puzzle remains why this effect is only modest.
The psychological impact of breast cancer diagnosis and its treatment was long neglected and the studies in the United Kingdom were perhaps pioneering in terms of elucidating the psychological and social effects of a mastectomy compared with breast-conserving surgery, or more recently, axillary clearance compared with sentinel node biopsy.2-4
The low sensitivity of clinical examination or any other imaging modality to accurately screen out the negative axilla meant that many patients were having their uninvolved axillary tissue excised unnecessarily along with its posttraumatic morbidity such as lymphedema, numbness, and shoulder restriction. This was demonstrated with a high level of objective evidence by the Edinburgh group,5 which also championed the concept of a formal 4-node axillary lymph node sampling. In their hands there was no false negative sample and the randomized trial confirmed that the procedure was safe and effective.6-8 However, this procedure has not become popular, mainly because of the lack of standardization outside of the Edinburgh group, which is partly inherent in the nature of the procedure. Now it appears to have been overtaken by events, although adaptation to the blue dye–guided 4-node sample9 appears to be an acceptable technique in those centers who do not have access to nuclear medicine services.
The elegant concept of the sentinel node—the existence of a “first” lymph node to drain lymph from the breast—was an anatomical boon to surgeons. It now appeared that at least in part, William Halsted and Handley were right in suggesting the systematic centrifugal march of cancer away from the breast. It was subsequently shown that in at least 90% to 95% of cases, breast cancer behaves in such a systematic manner that we can trace its path using a combination of a technetium labelled radiocolloid and a blue dye injected in the breast. Would it be safe to accept this 5% to 10% false-negative rate? A mathematical model suggests that the potential harm is unlikely to be clinically significant.10 However, it is considered essential to test the safety of only removing this lymph node in a randomized trial. A randomized trial (N = 298) was launched in Cambridge in November 1999, and reported11 that Sentinel Lymphy Node Biopsy (SLNB) in patients undergoing surgery for breast cancer results in a significant reduction in physical and psychological morbidity. The larger multicenter ALMANAC (Axillary Lymphatic Mapping Against Nodal Axillary Clearance) trial was launched in the United Kingdom (N = 1031, started in November 1999).12-14 The unique nature of this trial was the audit phase12 during which each center had to prove their ability to reliably detect the sentinel node. There have been associated techniques developed in the United Kingdom for needleless injection15 of the radiocolloid as well as for intraoperative rapid diagnosis using touch imprint cytological diagnosis16 and molecular biology techniques to detect the presence of cytokeratin-19 and mammaglobin mRNA.17 These trials have resulted in 2 major changes in clinical practice: (1) that it is practically possible to avoid morbidity of axillary clearance using the technique of sentinel node biopsy and (2) establishment of a unique standardized training and accreditation program for a specific new procedure in surgical oncology. It is reasonable to deduce from the false-negative rate in the ALMANAC trial and mathematical modelling that the false negativity in the procedure is unlikely to be harmful. However, we should be aware that data on recurrence or ultimate survival are yet to come, and while learning the procedure patients should be properly consented with the information about its pros and cons. Reassuring results of these trials means that the local therapies are the least disruptive and women are able to lead a nearly normal life following treatment of breast cancer.
Breast cancer was one of the first to be recognized as having a hormonal influence (Table 46-1). Traditionally, the credit for this has been accorded to George Beatson after he wrote in the Lancet in 1896.18,19 In 1876, young George Beatson (1848–1933) was asked to take medical charge of a man whose mind was affected, and went to reside with him at one of his estates in the west of Scotland. As his duties were not onerous, he had a good deal of leisure time to himself and decided that this was a good opportunity for writing his doctor of medicine thesis. He decided the subject to be lactation having observed the weaning of the lambs on a large adjoining sheep farm. At that time, lactation was thought to be controlled from the brain. His studies convinced him that this could not be the case. He also observed that in some countries castration of lactating cows was used to control lactation. He felt that the microscopic changes in the breast during lactation are only a shade different from that seen in a cancer.
| Agent | Initiation | Age Group | Mechanism |
|---|---|---|---|
| Surgical oopherectomy | 1890s Beatson | Premenopausal | Complete removal of ovarian influence |
| Radiation to ovaries | 1948 Christie | Premenopausal | Ablation of ovarian function by irradiation |
| Tamoxifen | 1977 NATO | Pre- and postmenopausal | Competitive inhibition (poor agonist) of the ER receptor |
| Goserelin | 1985 ZIPP | Premenopausal | Temporary chemical ovarian ablation with luteinizing hormone releasing hormone agonist |
| Anastrozole | 1995 ATAC | Postmenopausal | Depleting the source of estrogen in postmenopausal women by inhibition of the aromatase enzyme |
Twenty years later, he was faced with a 33-year-old woman who had developed breast cancer during her pregnancy and lactation. After the first surgery the disease had quickly recurred on the chest wall. The only remedy that was being tried for such patients was thyroid extract to which this patient did not respond. Dr Beatson, having properly obtained the patient’s consent, performed a bilateral oophorectomy that resulted in a long-lasting (4 years) response. In his second case he only had a partial response and in the last case described in the Lancet paper, he did not perform the operation because the patient was postmenopausal and he only gave her thyroid extract. As there was no response in her he concluded that thyroid extract does not have a beneficial effect, while oophorectomy was likely to be beneficial. It is remarkable that it was these 2 cases that inspired the conceptual leap that breast cancer is controlled in a nonneural manner by the ovaries. Stanley Boyd, an English surgeon, published his first 3 cases on October 2, 1897.20 Importantly, he also used this technique as an adjuvant therapy. His working hypothesis was that “internal secretion of the ovaries in some cases favours the growth of the cancer”20 and indicated that one-third of breast cancer patients clearly benefited from this approach.21 His patients were included in the series of 99 cases reported in 1905.22 The morbidity of the operation was the main stumbling block in the popularization of this approach and it took another 50 years before this concept was tested for the first time in a randomized trial, using nonsurgical methods.
The first trials to test the effectiveness of ovarian ablation either by radiotherapy or by chemical castration were initiated in the United Kingdom. The randomized trial (N = 189), testing the role of ovarian ablation with radiotherapy, was initiated at the Christie Hospital in Manchester in 1948.23,24 In this trial, 450 cGy radiotherapy was used for stopping the ovarian function and it was followed by many other ovarian ablation trials. In 1992, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) analyzed these ovarian suppression trials and demonstrated, contrary to the general opinion at the time, that ovarian suppression by any means improved survival from breast cancer.25 The Cancer Research Campaign-Under 50s UK trial, testing the effectiveness of chemical ablation using goserelin, was started in 1987.26 Goserelin is an agonist of the leutinizing hormone-releasing hormone (LHRH) and effectively blocks its effect, inducing an artificial temporary menopause. The ZIPP study (Zoladex in Premenopausal Patients) was born out of collaboration between the United Kingdom, Sweden, and Italy and compared in a 2 × 2 factorial design whether addition of goserelin improves disease-free and overall survival when added to standard adjuvant treatment including chemotherapy. The results were unequivocal. After a median follow-up of 5.5 years, ovarian suppression with goserelin for 2 years was well tolerated and improved disease-free survival (hazard ratio [HR] 0.80; 95% CI 0.69–0.92; p = .002) and overall survival (HR 0.81; 95% CI 0.67–0.99; p = .038) of premenopausal women.
ICI46474, which was to be later called tamoxifen, was developed by the ICI laboratories as an anti-estrogen contraceptive; it was a partial agonist of estrogen. The first presentation of its clinical effects on breast cancer was presented by Mary (“Moya”) Patricia Cole,27 from the Christie Hospital, Manchester Professor Michael Baum used it in advanced disease and found that it was extremely well tolerated. Being quite convinced of the need to use an “adjuvant” therapy to reduce mortality from breast cancer, he felt that this could be an ideal drug for prolonged use and that it should be tested in a randomized trial. This led to the establishment of the multicenter Nolvadex Adjuvant Tamoxifen Organization (NATO) study (N = 1285) that began recruitment in November 1977. In those days, the general feeling was that hormone therapy merely delayed death without actually reducing mortality. The laboratory evidence that tamoxifen improved disease-free survival after breast cancer in laboratory mice was presented in 1982 at the Surgical Research Society meeting by Mr Alan Wilson.28 The NATO trial was the first study to report a survival benefit from adjuvant tamoxifen in 1985.29-32
The Scottish adjuvant tamoxifen trial was started in 1978 and reported a similar benefit.33 The effect of these United Kingdom trials was that tamoxifen was adapted as adjuvant treatment in the United Kingdom long before the rest of the world; consequently, the beginning of the fall in breast cancer mortality was first seen in the United Kingdom.
The data from the Scottish trial have been recently used to validate a new mathematical method of estimating what proportion of patients actually benefit from adjuvant therapy (the V-G equation).34 By merely looking at the conventionally expressed results of a positive randomized clinical trial, it cannot be ascertained whether the additional benefit is distributed to all patients or limited to only a subgroup. We devised a new variance-guided equation to estimate the proportion (p) of the patients in whom a treatment is effective: p = 1/(1 + [(v(T) − v(C))/(s(T) − s(C))2]), where v = variance; s = survival; T and C = logarithms of survival times of treated and control groups; variance = (number of events) × (standard error)2. This equation was tested with the Scottish adjuvant tamoxifen trial (N = 1323). The trial included a significant number of patients with estrogen receptor (ER)–negative tumors who would not have derived any benefit from tamoxifen. Conveniently, 742 patients in this trial had their ER status ascertained and therefore could be used for validation. The new V-G equation independently predicted—only from the length of survival of individual patients—that 64% of patients in the Scottish trial benefited from tamoxifen, accurately predicting the proportion (60%−71%) of patients whose tumors were ER–positive. This vindication also supports a biologically plausible view that there is a subpopulation of patients among those treated who derive absolutely no benefit, while others may derive a variable amount of benefit. The equation can thus foretell the existence and frequency of a predictive factor (such as ER). Widely applicable to positive randomized clinical trials that have found an overall benefit from chemotherapy, hormone therapy, biologic therapy, or radiotherapy, this equation could suggest new biological and therapeutic insights and enable more precise patient consultation.
It should be noted that the NATO trialists did consider whether ER receptors were important predictors of response to tamoxifen. However, their data led them to conclude that it was probably not an important predictor.35 They were also heretical and eventually right in trying to use it among premenopausal women because tamoxifen actually increased estrogen levels in these women. They did raise the possibility that a longer duration of treatment may further improve the results. The maturation of the latter idea through other trial results has resulted in 2 other United Kingdom–led trials: ATLAS (Adjuvant Tamoxifen—Longer Against Shorter) and aTTom (adjuvant Tamoxifen Treatment—offer more).
ATLAS is a multicenter international trial led from the Clinical Trial Service Unit and Epidemiological Studies, Oxford. It is testing whether continuation of tamoxifen for 10 years rather than stopping at 5 years provides any additional benefit, without significant harm. The recruitment closed in March 2005, after randomizing 15,252 patients. The preliminary results were presented at the San Antonio Breast Cancer Conference in December 2007 (Peto R, Davies C, on behalf of the ATLAS Collaboration. International randomized trial of 10 versus 5 years of adjuvant tamoxifen among 11 500 women—preliminary results. 30th San Antonio Breast Cancer Symposium: Abstract 48) and suggested a 12% reduction in recurrence, by continuing tamoxifen beyond 5 years. The aTTom results were presented in the ASCO meeting in Chicago in June 2008,36 along with a meta-analysis of the 20,000 patients in such trials (ATLAS, aTTom, Scottish, ECOG, and the NSABP-14 trials). This suggested a 10% reduction (OR = 0.90, 95% CI 0.84–0.98, p = .01) in recurrence of breast cancer by taking 10 years of tamoxifen compared with 5 years. However, this needs to be balanced against the side effects and the current standard duration remains 5 years.
The preventive effect of tamoxifen was first suggested from the data in the CRC-2 trial.37 This was a 2 × 2 factorial design trial that tested the benefit of tamoxifen and chemotherapy, alone or in combination. From these data it was spotted that while there was no difference between the chemotherapy arms, tamoxifen significantly reduced the risk of new contralateral breast cancer, prompting the concept of chemoprevention.38
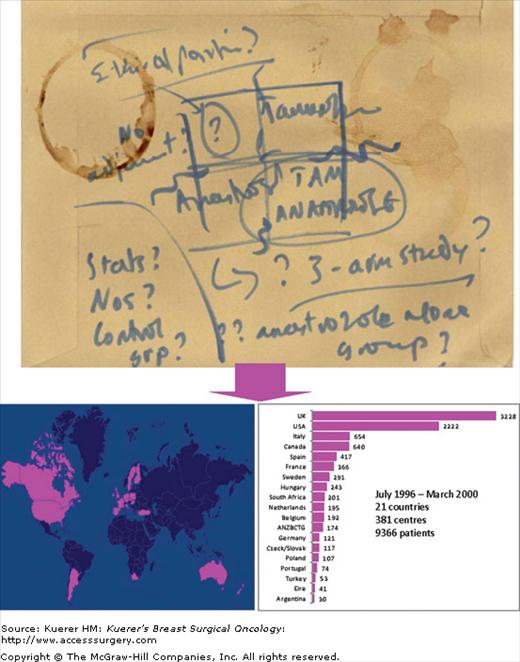
Tamoxifen is a remarkably effective drug and has few side effects, and it was many years before an alternative emerged. Anastrozole is an inhibitor of the aromatase enzyme that is essential for peripheral conversion of androgens to estrogen and is responsible for the main source of estrogen in the postmenopausal woman. The ATAC trial was born, literally, on the back of an envelope (Fig. 46-1) during a discussion between Prof. Michael Baum, Prof. Jeffrey Tobias, and Prof. Mitch Dowsett. The ATAC trial compared anastrozole and tamoxifen, alone or in combination, as adjuvant treatment for breast cancer. This trial eventually became the largest adjuvant therapy trial in the world, with 9366 patients randomized into the 3 arms of the trial. The early analysis of the data showed that the patients in the combination arm did not derive any additional benefit, while suffering from the side effects of both the drugs, so this arm has been unblinded and omitted from later analyses. The latest analysis at 100 months follow-up39 confirmed that the use of anastrozole reduced the risk of relapse—local and distant—and the risk of contralateral breast cancer. However, there was no reduction in overall mortality and it has been suggested that in this aging population, the small difference in mortality may be overshadowed by competing causes of death.
There were several spinoffs from this trial. For example, the Impact study was one of the few studies that could demonstrate that the effectiveness of a drug during the perioperative window to change certain biological parameters (Ki-67 in this case) may predict the ultimate effectiveness of a drug.40-43 Also, anastrozole was found to reduce the incidence of contralateral breast cancer, leading to the chemoprevention trial IBIS-II,44-47which is comparing anastrozole to placebo.
Older women have been perceived to have less aggressive disease and are frequently not fit enough to undergo surgery. This idea prompted many clinicians to use tamoxifen as the primary therapeutic measure and many observational studies yielded positive findings. These findings prompted 3 randomized trials (2 in the United Kingdom48,49 and an EORTC trial50) with the belief that tamoxifen alone may be able to control the disease through a woman’s natural lifespan. These trials did not show any survival benefit of surgery, although of course there was a clear benefit of local control. When the clear benefit of adjuvant tamoxifen was shown in the EBCTCG overview, 3 further trials were designed, again—1 European (Group for Research on Endocrine Therapy in the Elderly, GRETA51) and 2 in the United Kingdom: the Cancer Research Campaign (CRC)52,53 and the Nottingham trial.54 In the latest analysis of the CRC trial it was clearly shown that although tamoxifen is able to control the disease effectively for up to 3 years, breast cancer mortality, overall mortality, and local control are all better when surgery is added to the treatment schedule. This again added to the accumulating data challenging the idea that local control does not influence survival outcome. However, all these studies used 70 as an age cutoff. Even at the time of the trial initiation, the life expectancy at age 70 was 13 years. Tamoxifen alone was unlikely to have long-term control. Only 1 trial used ER receptor to select patients. However, the survival curves did not diverge for the first 3 years, despite having an unselected population in the CRC trial.
Today, we have a large number of older patients who are living longer with several comorbidities. We have drugs better than tamoxifen that are just as easy to use. In the NSABP P024 trial, the overall response rate was 55% for letrozole and 36% for tamoxifen (p < .001). Time to progression (in advanced disease) is longer for letrozole—about 1.5 times longer. At 80, the life expectancy is 89, and at 86 it is 92. Looking at it another way, on average, overall survival of women aged 80+ with breast cancer is 72% at 1 year and 58% at 5 years i.e., an estimated 65% at 3 years. Therefore, it is plausible that the hormonal treatment available today may provide good control for this period in a significant enough proportion of women to justify its use as the first-line treatment. This is the subject of the ESTEEM/PAINLESS trials that are hoping to start recruitment soon. The recent data from the ABCSG-12 study provides support for the addition of zoledronic acid to the medical treatment arm as it may further extend the duration of control.
Many of the early trials of chemotherapy, albeit not the first, were started in the United Kingdom. In fact, 10 of the 47 trials included in the 1995 overview were from the United Kingdom,55 providing 21% of patients. More recently, the United Kingdom contributed significantly to the multicenter HERA trial,56 which exemplified how a biological therapy is brought in successive steps from the bench to the bedside.
The UK trials (the St George’s Hospital, Scottish, West Midlands, and the CRC) contributed to the evidence in the EBCTCG overview57 that radiotherapy after breast-conserving surgery (BCS) was essential and that without it the risk of local recurrence as well as mortality was significantly increased by 29% and 17%, respectively. A note of caution was also published for the first time from the Kings/Cambridge trial. A 10-year analysis of this trial demonstrated a higher risk of death from other causes (cardiac and other cancers) in patients who received radiotherapy after a simple mastectomy on the left side.58
By the 1990s the clinical dogma created by the new evidence59,60 was that BCS plus whole-breast fractioned radiotherapy is the gold standard of treatment. This approach, although “conservative” in name, is still “radical” in spirit—its intent is the same as that of the major extirpative surgery performed by William Halstead over 100 years ago,61 while we are faced with the irony of offering radical local therapy to patients with smaller and smaller tumors. As we stand on these giants’ shoulders, the next step—the real paradigm shift—to a local therapy truly localized to the tumor and its environs in selected patients might be easier.
The United Kingdom has been home to trials of partial breast irradiation. In the Christie hospital trial,62 708 patients were randomized to receive either the standard wide field (WF) radiotherapy or a limited field (LF) radiotherapy to the index quadrant. Overall, there was a higher recurrence rate in the latter (LF) arm. In the limited field arm, a constant size of radiotherapy field was used, irrespective of the tumor size, and this could have resulted in several instances of “geographical misses.” More importantly, when the results were analyzed according to the type of the primary tumor, it was found that limited field radiotherapy was inadequate only in infiltrating lobular cancers or cancers with extensive intraductal component (EIC). In the 504 cases of infiltrating duct carcinoma, there was no significant difference in the local recurrence rates of the 2 arms.
There were also 2 smaller studies of patients given partial breast irradiation with interstitial implants from the Guy’s Hospital and the Ninewells Hospital in Dundee, Scotland.63 None of the patients in the Scottish series (N = 11) had recurred at the time of publication (median follow up 5.6 years), and in the Guy’s Hospital series (N = 27)64 a single continuous application of an iridium-192 implant delivering 55 Gy over 5 to 6 days replaced the standard radiotherapy regimen including whole-breast radiotherapy plus tumor bed boost. The authors found a 20% increase in local recurrence compared with historical controls. However, as discussed in a letter in response to the study,65 it was pointed out that the biologically effective dose (BED) was 20% lower than conventional radiotherapy and this almost completely explained the difference. In addition 12 out of the 27 patients were node positive and 15 out of the 27 had involved margins, putting these patients at high risk of local recurrence already.
Mastectomy rates around the world vary widely, being influenced by local culture, distance from radiotherapy facilities, the surgeon’s choice, and the patient’s preferences, not necessarily in that order. For example, within the ATAC trial, the mastectomy rates were 42% in the United Kingdom versus 51% in the United States. The perceived need for having a prolonged course of postoperative radiotherapy is a major barrier against wider acceptance of breast-conserving therapy for several reasons. It adds yet another tiresome 3- to 6-week course of radiotherapy, requiring daily hospital visits, for patients who may already have had a 6- to 9-month course of chemotherapy. The radiotherapy schedule is inconvenient for patients and contributes substantially to the unacceptable waiting lists experienced in many oncology departments worldwide. Many women are forced to choose mastectomy because they live too far away from a radiotherapy facility or have difficulty travelling to one. Even many patients treated with BCS may not receive optimal treatment because of living too far from a radiotherapy center. One study in the United States found that when the travel distance was less than 10 miles, 82% of patients received radiotherapy after BCS; when it was 50 to 75 miles, 69% received it; and when it was more than 100 miles, only 42% received it.66 (The proportions of patients in these 3 groups receiving BCS including radiotherapy were 39%, 22%, and 14%, respectively.) Further, in countries with scarce radiotherapy resources, patients treated with BCS may wait a prolonged time before beginning radiotherapy. Another study67 of 7800 patients suggests that delaying the initiation of conventional radiotherapy for 20 to 26 weeks after surgery was associated with decreased survival. The delay imposed by giving chemotherapy before radiotherapy might also increase the risk of local recurrence. When making decisions about which operation to choose, recurrence, radiation therapy, and quick recovery are the main factors women are concerned about.68 Consequently, if radiation can be completed at the time of the surgery then 2 large concerns will be taken care of and perhaps fewer women will feel obliged to choose mastectomy just because they live far away from a radiotherapy facility66 or to avoid prolonging their treatment.
It has been estimated that the externally delivered boost dose misses target volume in 24% to 88% of cases.69,70 Thus, a large proportion of local recurrences could be attributed to this “geographical miss.” This could be even more important today in the age of oncoplastic surgery when there is extensive remodelling of the breast performed to achieve a better cosmetic result. In this situation, it is very difficult to delineate the tumor bed even with markers such as gold seeds. This can result either in complete missing of the target or a “precautionary” overtreatment by enlargement of the boost field. Delivering radiotherapy soon after tumor excision with the TARGIT approach, before remodelling occurs, could ensure that the radiotherapy (boost or alone) is delivered to the correct target.
A delay in delivery of radiotherapy, either because of a long waiting list or because chemotherapy is given first, may jeopardize its effectiveness,67,71 though this has been difficult to substantiate. I believe that the really important delay may, however, be the one that occurs immediately after surgery. We have found that the tumor bed is a rich microenvironment that promotes proliferation, migration, and invasion.72-74 Targeting this microenvironment at the right time could be crucially important. I would like to call missing this window of opportunity a “temporal miss” analogous to its spatial counterpart. Finally, whole-breast irradiation carries the risks of acute and long-term complications such as erythema, fatigue, prolonged discomfort, radiation pneumonitis, rib fracture, cardiovascular effects, and carcinogenesis that could compromise the long-term benefit from postoperative radiotherapy.60,75
Recent molecular biology data provide more evidence for the concept of a field defect. The morphologically normal cells surrounding breast cancer demonstrate a loss of heterozygosity, which is often identical to that of the primary tumor.76 In addition, aromatase activity in the index quadrant is higher than other quadrants77 and via estrogen has the potential to stimulate mutagenesis, growth, and angiogenesis.78,79 Patients with ipsilateral breast tumor recurrence (IBTR) have an increased risk of carrying the mutant p53 gene (23% vs 1%),80 and young patients (<40 years) with IBTR have a disproportionately increased risk (40%) of carrying a deleterious BRCA1/2 gene mutation.81 This suggests that local recurrence is probably related more to background genetic instability than to different tumor biology at a younger age. It appears that a dynamic interaction between the local factors (such as aromatase) present in the breast parenchyma, the systemic hormonal milieu, and genetic instability will determine the risk of local recurrence, in addition to the biology of the excised primary tumor.
The location of recurrence in the breast with respect to site of the primary tumor shows an interesting distribution. Between 80% and 100% of early breast recurrences occur in the quadrant that had the primary tumor, which is in contrast to the findings of 3-dimensional (3D) analysis of mastectomy specimens,82 which reveals that 63% of breasts harbor occult cancer foci and 80% of these are situated remote from the index quadrant. It therefore appears that these widespread and occult multifocal/multicentric cancers in other quadrants of the breast remain dormant for a long time and have a low risk of causing clinical tumors. This is corroborated by the fact that although there is a high frequency (20% in young [median age 39] women and 33% in women between 50 and 55) of tumors found in breasts when analyzed in autopsy studies,83 the frequency of clinical breast cancer in the population is considerably lower.
Arguably, in the EORTC study84 only 56% of local recurrences are reported to have occurred in the original tumor bed. In fact, a further 27% recurred diffusely throughout the breast including the tumor bed, leaving 29% of recurrences outside the index quadrant. However, patients in this study received intensive mammographic follow-up, which may have unearthed subclinical occult tumors in other quadrants of unproven clinical significance.
It is remarkable that most early local recurrence occurs in the index quadrant, whether or not radiotherapy is given85-87 and irrespective of clear margins. Of the breast-conserving trials that have tested the effect of radiotherapy, patients in the NSABP-B06,88 Ontario,89 Swedish,90 and Scottish91 trials had less extensive surgery compared with the Milan III trial.92 The recurrence rate in the control arm of the Milan III trial, in which the tumors were smaller and excision was considerably wider, was low (8.8% vs 24%-27% in other trials) albeit at the cost of cosmesis. Nevertheless, radiotherapy reduced it even further and at the same proportional rate as in other trials. If local recurrence were caused by residual disease only, then radiotherapy should have effected a much larger proportional reduction in those patients with positive margins or less extensive surgery; but radiotherapy is as effective in patients with negative margins, suggesting that radiotherapy may have an effect on the soil rather than the seed.93
Thus, radiotherapy may have a dual effect of inhibiting the growth of genetically unstable cells around the primary tumor and of making the whole breast tissue less conducive to growth.93 This idea has been supported by translational research in patients undergoing intraoperative radiotherapy (IORT). A study performed at the Centro di Riferimento Oncologico, Aviano, Italy,74 demonstrated for the first time that radiotherapy could be exerting its beneficial effect via an effect on the tumor microenvironment. We found that the wound fluid collected in the 24 hours following surgical local excision of cancer stimulates breast cancer cell lines to proliferate, migrate, and invade into Matrigel. On the other hand, the fluid collected from wounds that had received targeted IORT did not have such an effect. Thus, if radiotherapy is delivered immediately after the operation using the TARGIT approach, it could be superior to the conventional radiotherapy that suffers from what I call a “temporal miss.”
There has been considerable evidence suggesting that surgery may perturb the hormonal milieu in a deleterious manner.94-98 If IORT is able to locally change the composition of the wound fluid, which essentially derives from the peripheral serum, it is not completely inconceivable that there could be a measurable and important systemic effect of such a treatment. Alternatively, if the mechanism of action of these effects is elucidated with further studies, we may be able to replicate it with a systemic agent that can counter the ill effects of surgery.
Systemic therapies such as aromatase inhibitors or ovarian suppression may achieve a similar effect on the microenvironment through reduction of estrogen concentration in the breast and may have a synergistic effect with radiotherapy.99 Thus, with increasing use of systemic therapy, IORT to the tissues surrounding the primary tumor might be all that is necessary and such an approach may solve many of the problems of postoperative radiotherapy discussed earlier and may allow many more women with breast cancer to conserve their breast.
The main basis of IORT is that a single dose of IORT could have a biological effect on tissue that is equivalent to a full course of fractionated external beam radiotherapy (EBRT). This is therefore being tested in randomized trials. There is already some evidence suggesting the safety and effectiveness of a single dose of radiotherapy in achieving tumor cell kill.93,100-102 The theoretical basis for calculation of the biological effects of a given dose of radiation is the linear-quadratic (LQ) model. This model is based on the different shapes of cell survival curves of acute- and late-reacting tissues. It is assumed that large single doses of radiation are more effective on late-responding tissues as compared to acute-reacting tissues. However, the LQ model is reliable for single doses up to 6 to 8 Gy only and may therefore not be appropriate for modelling the effects of higher single doses (~20 Gy), which are used in IORT or radiosurgery.
A detailed analysis of the radiobiological aspects specific to the Intrabeam system is available elsewhere.103-105 As regards toxicity, the thickness of the chest wall should ensure that virtually no risk of pneumonitis is expected. The same is true for the heart. Since the dose to the heart and lungs during IORT is almost negligible, the mortality from cardiac ischemia that has been observed in some trials using conventional radiation therapy75,106-108 should not be seen. The calculated low risk of toxicity is in good agreement with the available clinical data from patients treated with TARGIT.101,109-112 The single-dose radiation using Intrabeam is administered over 25 to 35 minutes. Since normal tissues can repair their DNA within a few minutes, a large proportion of radiation-induced DNA damage is repaired in normal tissues during this long duration of IORT. On the other hand, cancer cells or precancerous cells with poor DNA-repair machinery are unable to do so. Thus, radiation using Intrabeam administered over 25 to 35 minutes would have a high therapeutic index, and would induce less normal tissue damage than similar doses given over 2 to 3 minutes113,114 as used when electrons are employed (ELIOT trial).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree