The Thyrotropin Receptor
Gilbert Vassart
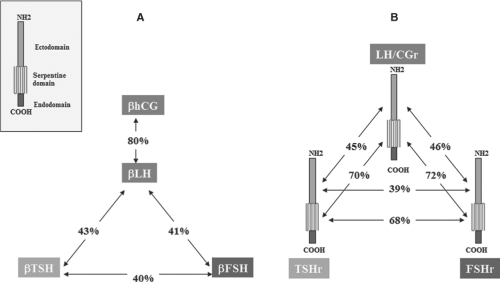
Thyrotropin (TSH) is a heterodimeric glycoprotein hormone composed of two subunits, an α subunit that is common to TSH, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and chorionic gonadotropin (CG), and a unique β subunit. The β subunits, which confer to these hormones their specific biological activity, are encoded by paralogous genes and, as such, display structural similarity (Fig. 10B.1). The corresponding receptors for TSH, FSH, and LH/CG also have structural similarities and are encoded by paralogous genes. All three receptors are mainly coupled to cyclic AMP (cAMP) generation via Gαs, with additional coupling to Gαq, when exposed to high concentrations of their agonist.
The Structure of the Thyrotropin Receptor
The receptors for TSH, FSH, and LH/CG are members of the rhodopsin-like G-protein–coupled receptor (GPCR) family. As such, the TSH receptor has a “serpentine” domain containing seven transmembrane regions with many (but not all) of the features typical of this receptor family. In addition, and a hallmark of the subfamily of glycoprotein hormone receptors (1,2,3), it has a large (about 400 amino acid residues) amino-terminal extracellular domain that contains sites that selectively bind TSH with high affinity (4). The higher sequence identity of the serpentine domains of the glycoprotein hormone receptors (about 70%), as compared with the extracellular domains (about 40%, Fig. 10B.1) suggests that the former are interchangeable modules capable of activating guanine-nucleotide-binding (G-) proteins (mainly Gαs) after specific binding of the individual hormones to the latter (5). Contrary to other rhodopsin-like G-protein–coupled receptors, the glycoprotein hormones bind to their respective extracellular domains with high affinity in the absence of the serpentine domain (6,7,8). The intramolecular transduction of the signal between these two portions of the receptors involves a still incompletely defined mechanism specific to the glycoprotein hormone receptor family (see below). The relatively high sequence identity between the hormone-binding domains of the TSH and LH/CG receptors opens the possibility of spillover phenomena during normal or, even more so, molar, or twin pregnancies, when serum CG concentrations are several orders of magnitude higher than are serum TSH concentrations. This provides an explanation to cases of gestational thyrotoxicosis (see later, see Chapters 21 and 56).
The TSH receptor contains six sites for N-glycosylation, of which four are effectively glycosylated (8). The functional role of the individual carbohydrate chains is still debated. It is likely that they contribute to the routing and stabilization of the receptor as it passes through the membrane system of the cell and is inserted into the cell membrane. Alone among the glycoprotein hormone receptors, the extracellular domain of the TSH receptor is cleaved, severing it from the serpentine domain (9). This phenomenon has been related to the presence in the extracellular domain of the receptor of a 50 amino acid insertion for which there is no counterpart in the FSH receptor or LH/CG receptor. The initial cleavage step, due to the action of a metalloprotease, takes place at around position 314 (within the 50 amino acid insertion) from the amino terminus of the receptor, and is followed by removal of approximately 50 amino acids from the amino-terminal end of the serpentine-containing portion of the receptor (10,11). The amino-terminal end of the receptor remains bound to the extracellular end of the serpentine domain by disulfide bonds. The functional importance of this TSH receptor-specific posttranslational modification remains unclear. While all wild type TSH receptors on the surface of thyroid follicular cells seem to be in cleaved form, non-cleavable mutant constructs are functionally indistinguishable from cleaved receptors, when expressed in transfected cells (9). Residues 303 to 366 can be deleted from the hinge region with minimal effects on the function of the receptor (12). When transiently or permanently transfected in non-thyroid cells, wild type human TSH receptors are present at the cell surface as a mixture of monomers and cleaved dimers. There are indications from immunization experiments in mice that cleavage and possible shedding of the amino-terminal portion of the receptor would play a role in the generation of stimulating autoantibodies in patients with Graves’ disease (13,14) (see Chapter 18A).
The TSH receptor is specifically inserted into the basolateral membrane of thyroid follicular cells. This phenomenon involves a signal (amino acids 731 to 746) unusually localized in the very C-terminal portion of the receptor, at a marked distance from the membrane (15).
The possibility that TSH receptors are present on the cell surface as dimers of cleaved dimers was raised after demonstration that most rhodopsin-like G-protein–coupled receptors do dimerize (16). Functional complementation of TSH receptors with mutations in the extracellular and the serpentine domains has been observed after expression of receptor constructs in transfected cells (17). A definitive demonstration that glycoprotein hormone receptors do dimerize in vivo and that dimers interact functionally has been provided by similar complementation experiments performed in LH/CG receptor knockout mice. Mice co-expressing two inactive mutant receptors are fertile (18). Direct demonstration of dimerization of the TSH receptor has been provided by Bioluminescence Resonance Energy Transfer (BRET) and the dimers have been shown to display negative cooperativity for binding of TSH (17). Whether this allosteric behavior of the receptor has (patho) physiologic significance remains to be determined.
The Thyrotropin Receptor Gene
The gene coding for the human TSH receptor is located on the long arm of chromosome 14 (14q31) (19,20). It is organized into 10 exons. The extracellular domain is encoded by a series of 9 exons, each of which corresponds to one or an integer number of leucine-rich repeat segments (see later). The carboxyl-terminal half of the receptor containing the carboxyl-terminal part of the extracellular domain and the serpentine domain is encoded by a single large exon (21), in keeping with the fact that the genes for many G-protein–coupled receptors have no introns. A likely evolutionary scenario derives from this geneorganization: The glycoprotein hormone receptor genes would have evolved from the condensation of an intron-less classic G-protein–coupled receptor with a mosaic gene encoding a protein with leucine-rich repeat segments (21). Triplication of this ancestral gene and subsequent divergence led to the receptors for TSH, FSH, and LH/CG. The existence of 10 exons in both the TSH and FSH receptor genes (as opposed to the 11-exon LH/CG-receptor gene), suggests the following evolutionary steps: First, duplication of an ancestral glycoprotein hormone receptor gene, yielding the LH/CG-receptor gene and the ancestors of the TSH- and FSH-receptor genes. After losing one intron, the latter duplicated subsequently into the TSH- and FSH-receptor genes. The family of the glycoprotein hormone receptors comprises five additional members presenting a similar pattern made of leucine-rich repeats in the ectodomain, upstream of a rhodopsin-like serpentine domain: Two of them (LGR7, LGR8) encode insulin-like 3 and relaxin receptors, respectively (22), the remaining three (LGR4, LGR5, LGR6) are orphan receptors involved in the regulation of epithelial stem cell biology (23).
The promoter of the TSH receptor gene has the characteristics of a housekeeping gene promoter, being GC-rich and devoid of a TATA box. In rats it stimulates transcription from multiple start sites (24) and it contains a functional thyroid transcription factor-1 (TTF-1) recognition site (25). Expression of the TSH-receptor gene is largely thyroid-specific. Constructs made of a chloramphenicol acetyl transferase reporter gene under control of the 5′-flanking region of the rat TSH-receptor gene are expressed when transfected into FRTL5 cells and FRT cells but not into non-thyroid HeLa or rat liver (BRL) cells (24). However, TSH receptor mRNA has been clearly demonstrated in fat tissue of guinea pigs (26), in adipocytes (27,28) and in ependymal cells of the mediobasal hypothalamus in the mouse, where it plays a role in adjustment of reproductive physiology to the length of the days (29,30). TSH receptors may also be present in lymphocytes, extraocular tissue, cartilage, and bone, but their functional importance in these tissues is uncertain (31,32). Expression of the TSH receptor in thyroid cells is extremely robust. It is moderately upregulated by TSH in vitro and downregulated by iodide in vivo (33).
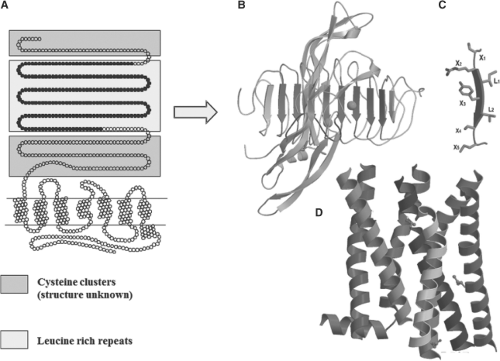 Figure 10B.2 Schematic representation of the structure of the TSH receptor. A: Two-dimensional representation with indication of the various domains. The blue boxes correspond to amino-terminal and carboxyl-terminal cysteine-rich portions of the extracellular domain, flanking leucine-rich repeats (LRR, yellow box). B: General view of the follicle-stimulating hormone receptor (FSHr)–FSH crystal structure as a template to model the interaction between TSH and the TSH receptor (41). The concave inner surface of the receptor, formed by ten leucine-rich repeats (LRR2–9, shown in blue), contact the middle section of the hormone molecule, both the C-terminal segment of the α subunit and the “seat-belt” segment of the β-subunit (shown in red). C: Each LRR is composed of the X1-X2-L-X3-L-X4-X5 residues (where X is any amino acid, and L usually is Leu, Ile, or Val), forming the central portion (X2-L-X3-L-X4) of a typical beta-strand, whereas X1 and X5 are parts of the adjacent loops. D: Molecular model of the transmembrane domain of the TSH receptor, constructed from the crystal structure of bovine rhodopsin. The color code of the α-carbon ribbons is: Transmembrane helix 1 (crimson), 2 (golden red), 3 (dark red), 4 (gray), 5 (red), 6 (orange), and 7 (blue), and helix 8 (blue). The structures of available class A rhodopsin-like GPCRs are similar at the transmembrane domain. See color plate. |
Recognition of the Receptor by Thyrotropin
The three-dimensional structures are available for hCG and FSH (34,35,36) which allows accurate modelization of TSH on these templates. The crystal structure of the human FSHr–FSH
complex (37) has confirmed that the ectodomain of glycoprotein hormone receptors belongs to the family of proteins with leucine-rich repeats (LRRs) (38). The concave inner surface of the receptor (Fig. 10B.2) is an untwisted, non-inclined beta-sheet formed by 10 LRRs. While the N-terminal portion of the beta-sheet (LRR1–7) is nearly flat, the C-terminal portion (LRR7–10) has the horseshoe-like curvature of LRR proteins. The crystal structure of part of the TSHr ectodomain in complex with thyroid-stimulating or thyroid-blocking autoantibodies has recently been obtained (6,39,40). Notably, both the structure of the ectodomain of TSHr and the receptor binding arrangements of the autoantibodies are very similar to those reported for the FSHr–FSH complex. The ectodomain of glycoprotein hormone receptors also contains, downstream of the LRR region, a cysteine cluster domain of unknown structure (the hinge region), involved in receptor inhibition/activation and containing sites for tyrosine sulfation important for hormone binding (see later).
complex (37) has confirmed that the ectodomain of glycoprotein hormone receptors belongs to the family of proteins with leucine-rich repeats (LRRs) (38). The concave inner surface of the receptor (Fig. 10B.2) is an untwisted, non-inclined beta-sheet formed by 10 LRRs. While the N-terminal portion of the beta-sheet (LRR1–7) is nearly flat, the C-terminal portion (LRR7–10) has the horseshoe-like curvature of LRR proteins. The crystal structure of part of the TSHr ectodomain in complex with thyroid-stimulating or thyroid-blocking autoantibodies has recently been obtained (6,39,40). Notably, both the structure of the ectodomain of TSHr and the receptor binding arrangements of the autoantibodies are very similar to those reported for the FSHr–FSH complex. The ectodomain of glycoprotein hormone receptors also contains, downstream of the LRR region, a cysteine cluster domain of unknown structure (the hinge region), involved in receptor inhibition/activation and containing sites for tyrosine sulfation important for hormone binding (see later).
Replacement by site-directed mutagenesis of the residues facing the hormone in the leucine-rich repeat portion of the TSH receptor (Fig. 10B.2) with their counterparts in the LH/CG receptor has been performed to assess the structural bases of binding selectivity (4). Exchanging eight amino acids of the TSH receptor for the corresponding amino acids in the LH/CG receptor resulted in a mutant receptor that bound human CG as well as the wild-type LH/CG receptor. While gaining sensitivity to human CG, the mutant receptor retained normal sensitivity to TSH, making it a dual-specificity receptor. It is necessary to exchange 12 additional amino acid residues to fully transform this mutant receptor into a bona fide LH/CG receptor (4). From an evolutionary point of view, these observations indicate that the specificity of hormone receptors is based on both attractive and repulsive residues, and that residues at different homologous positions have been selected to this result in the different receptors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree