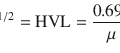7.1 Anatomy of the Thyroid Gland
The thyroid gland is located beside the trachea, just below the larynx. It has two oval lobes, one of each side of the trachea. The thyroid isthmus lies at about half distance between the thyroid cartilage and the sternal notch.
Laterally, from the anterior to posterior, the internal jugular vein, the vagus nerve, and the common carotid artery are in close relation to the thyroid, as it is possible to observe in the intra-operatory image of the thyroid bed, obtained after the removal of the thyroid gland (Fig. 7.1).
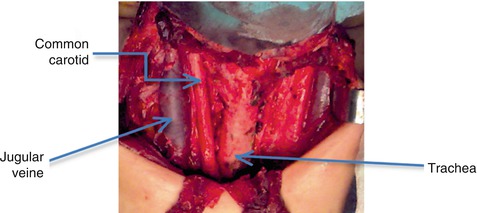

Fig. 7.1
Thyroid bed, after surgical thyroid removal (With the courtesy of A. Irimie, from the Institute of Oncology “Prof. Dr. I. Chiricuţă” Cluj-Napoca)
The parathyroid glands are also located in the posterior part; usually there are four: two in the upper bilateral part and two in the lower part of the thyroid.
The recurrent laryngeal nerves are also found in the posterior part, between the trachea and the thyroid. These are very important and delicate elements during the surgery of the thyroid (Fig. 7.2).
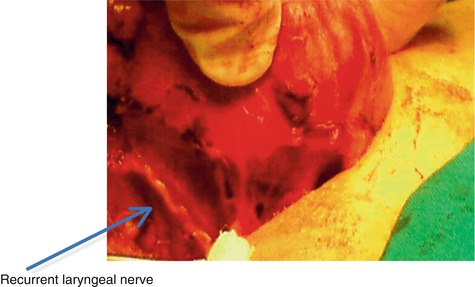

Fig. 7.2
Recurrent laryngeal nerve posterior of thyroid gland (With the courtesy of Dr. A. Irimie, Institute of Oncology “Prof. Dr. I. Chiricuţă” Cluj-Napoca)
The thyroid gland consists of two lobes (right and left) linked by a common part, the isthmus. From the upper margin of the thyroid isthmus may exist sometimes another accessory lobe, named Lalouette pyramid. This particular shape frequently associated with a “butterfly” will be easy to recognize during many of the nuclear imaging procedures.
As a special attention regarding the anatomy details, it is important to underline that, during intrauterine evolution, the thyroid gland descends from its upper position under the tongue, until it reaches the normal pretracheal position. Sometimes, there is a leftover structure in the thyroglossal duct, a failure in descend, which is able to lead to different pathologies involving thyroid tissues in this area.
The thyroid gland is highly vascularized, being mainly supplied from the superior and inferior thyroid arteries, which lie between capsule and pretracheal fascia (false capsule). All thyroid arteries anastomose with one another on and in the substance of the thyroid, but there is little anastomosis across the median plane (except for branches of the superior thyroid artery).
The superior thyroid artery belongs to the first branch of extern carotid artery, it descends to the superior pole of the gland, it pierces the pretracheal fascia, and then it divides into 2–3 branches. The inferior thyroid artery is a branch of the thyrocervical trunk, it runs superomedially posterior to the carotid sheath, it reaches the posterior aspect of the gland, and it divides into several branches, which pierce pretracheal fascia to supply the inferior pole of the thyroid gland. In approximately 10% of the people, the thyroid IMA artery arises from the aorta, the brachiocephalic trunk, or the internal carotid artery, and it ascends anterior to the trachea to supply the isthmus.
Usually, there are three pairs of veins that drain the venous plexus on the anterior surface of the thyroid: the superior thyroid veins drain its superior poles, the middle thyroid veins drain its lateral parts, and the inferior thyroid veins drain its inferior poles.
Lymphatics run in the interlobular connective tissue, often around arteries, communicate with a capsular network of lymph vessels, and after that pass to the prelaryngeal lymph nodes and to the pretracheal and paratracheal lymph nodes. Lateral lymphatic vessels along the superior thyroid veins pass to deep cervical lymph nodes.
The nerves derived from the superior, middle, and inferior cervical sympathetic ganglia reach the thyroid through the cardiac and laryngeal branches of the vagus nerve, which accompanies the arterial vessels; postganglionic and vasomotor fibers have indirect action on the thyroid by regulating the blood vessels.
The thyroid gland is made up of a large number of individual units, called thyroid follicles, and a number of larger cells, called parafollicular cells, which are responsible for the secretion of calcitonin.
Each follicle consists of a layer of follicular cells surrounding the colloid content, which gives a special histological appearance to the thyroid tissue.
The colloid has a variable content, according to the functional status of the thyroid gland. The size of the thyroid gland is largely variable in the range of normal values, being influenced by age, gender, area of residence, and iodine content of environment. Usually, in the case of adults, a thyroid lobe is 4–5 cm high, 2 cm wide, and 2 cm thick, and the thyroid can weigh about 20–30 g. The pathologies affecting the gland may enlarge it considerably, sometimes reaching a weight of 500 g.
7.2 Physiology of the Thyroid Gland
The role of the thyroid gland is the synthesis and release of the thyroxine (T4) and triiodothyronine (T3) thyroid hormones. Circulating iodide is actively transported into the cytosol, where a thyroid peroxidase oxidizes it and iodinates tyrosine residues on the thyroglobulin (Tg) molecule; iodination occurs mostly at the apical plasma membrane. A rearrangement of the iodinated tyrosine residues of thyroglobulin in the colloid produces the iodothyronines T4 and T3. Thyroid-stimulating hormone binding to receptors on the basal surface stimulates follicular cells to become columnar and to form apical pseudopods, which engulf the colloid by endocytosis. After the colloid droplets fuse with lysosomes, controlled hydrolysis of iodinated thyroglobulin liberates T3 and T4 into the cytosol.
These hormones are released into the bloodstream and lymphatic vessels. These processes are promoted by the thyroid-stimulating hormone (TSH), which binds to G-protein-linked receptors on the basal surface of follicular cells.
Parafollicular cells are also called clear (C) cells because they stain less intensely than thyroid follicular cells. They synthesize and release calcitonin, a polypeptide hormone, in response to high blood calcium levels.
The regulation of the thyroid function follows up the hypothalamic-pituitary-thyroid axis negative feedback mechanism (Fig. 7.3):
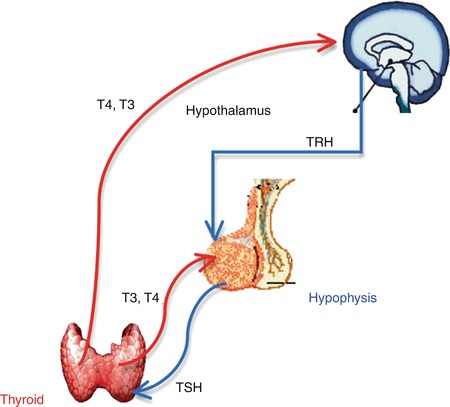

Fig. 7.3
The hypothalamic-pituitary-thyroid axis negative feedback mechanism
Thyrotropin-Releasing Hormone (TRH)
TRH is produced by the hypothalamus.
Release is pulsatile, circadian.
Travels through the portal venous system to the adenohypophysis.
Stimulates thyroid-stimulating hormone (TSH) formation.
Thyroid-Stimulating Hormone (TSH)
Upregulated by TRH
Downregulated by thyroid hormones
Travels through the portal venous system
Stimulates iodine uptake, colloid endocytosis, and growth of the thyroid gland
Produced by adenohypophysis
Biosynthesis of T4 and T3 Includes
Dietary iodine (I) ingestion.
Active transport and uptake of iodide (I−) by the thyroid gland.
Oxidation of I− and iodination of thyroglobulin (Tg) tyrosine residues.
Coupling of iodotyrosine residues to form T4 and T3; the iodination of tyrosyl residues forms monoiodotyrosine (MIT) and diiodotyrosine (DIT), which are then coupled to form either T3 or T4.
Proteolysis of Tg with release of T4 and T3 into the circulation.
Active Transportation and I− Uptake by the Thyroid
Dietary iodine reaches the circulation as iodide anion (I−).
The thyroid gland transports I− to the sites of hormone synthesis.
Accumulation in the thyroid is an active transportation process stimulated by the TSH.
Must be oxidized to be able to iodinate tyrosyl residues of Tg.
Both reactions are catalyzed by TPO.
Thyroperoxidase (TPO)
TPO catalyzes the oxidation steps involved in I− activation, iodination of Tg tyrosyl residues, and coupling of iodotyrosyl residues.
TPO acts in the proteolysis of Tg with release of T4 and T3.
Production of T4 and T3 and Conversion
T4 and T3 are synthesized and stored within the Tg molecule.
To liberate T4 and T3, Tg is resorbed into the follicular cells in the form of colloid droplets, which fuse with lysosomes to form phagolysosomes.
Tg is then hydrolyzed to T4 and T3, which are then secreted into the circulation.
T4 is the primary secretory product of the thyroid gland, being the only source of T4. The thyroid secretes approximately 70–90 μg of T4 per day; the total daily production rate of T3 is about 15–30 μg (80% of circulating T3 comes from deiodination of T4 in peripheral tissues, and 20% comes from direct thyroid secretion).
T4 is biologically inactive in target tissues until converted to T3; T3 then becomes the biologically active hormone responsible for the majority of thyroid hormone effects.
Sites of T4 Conversion
The liver is the major extrathyroidal T4 conversion site for the production of T3.
Some T4 to T3 conversions also occur in the kidney and other tissues.
Hormonal Transport
More than 99% of the circulating T4 and T3 is bound to plasma carrier proteins: thyroxine-binding globulin (TBG) binds about 75%; transthyretin (TTR), also called thyroxine-binding prealbumin (TBPA), binds about 10–15%; albumin binds about 7%; and high-density lipoproteins (HDL) bind about 3%.
The only unbound (free) hormone has metabolic activity and physiologic effects.
Free Hormones
Free hormones (FT3, FT4) are a tiny percentage of the total of hormones in the plasma (about 0.03% T4; 0.3% T3).
Increased TBG: increased levels of total serum thyroid hormones T4 and T3; free T4 (FT4) and free T3 (FT3) concentrations remain unchanged.
Decreased TBG: decreased levels of total serum T4 and T3—FT4 and FT3 levels remain unchanged.
From the above, it is apparent that the plasma concentrations of T4 and T3 are a function of TRH, TSH, thyroid production of T4 and T3, TBG, peripheral deiodinative conversion of T4 to T3, and deiodinative clearance of T3. Perturbation in any of these determinants may lead to changes in others, and these changes may be reflected by changes in thyroid function studies.
The Functions of the Thyroid Hormones
The thyroid hormone initiates or sustains differentiation and growth: it stimulates the formation of proteins; it is essential for normal brain development and essential for childhood growth; untreated congenital hypothyroidism or chronic hypothyroidism during childhood can result in incomplete development and mental retardation.
The thyroid hormone influences cardiovascular hemodynamics: increased cardiac output, increased heart rate and stroke volume, increased systolic pressure, and decreased systemic vascular resistance.
The thyroid hormone-mediated thermogenesis (increased expression of mitochondrial uncoupling proteins).
The thyroid hormone influences the female reproductive system.
The thyroid hormone is critical for normal bone growth and development.
The thyroid hormone regulates mitochondrial activity.
The thyroid hormones stimulate metabolic activities in most tissues: increased basal metabolic rate, increased oxygen consumption, and increased protein turnover.
With its features, the thyroid gland is the “internal clock” for all the functions of the body, a normal thyroid function being reflected in the whole equilibrium of the human organism. If we consider that an adult thyroid gland weighs about 30 g, this represents in a healthy 70 kg adult about 0.05% of all his body; but without a normal development of the thyroid gland, without this minimal quantity of special tissue, the passage from primitive human being to Homo sapiens would not be possible.
7.3 Diagnosis of the Thyroid Gland
7.3.1 Clinical Examination
Among other endocrine glands, the thyroid gland has a particular advantage related to its easy accessible situation. As previously mentioned, the malfunction of the thyroid gland may be reflected in every organ or system of the body, covering a multitude of signs and symptoms. This is the reason why every physician of any specialty must be prepared at least in performing a physical examination and in doing a careful anamnestic interview.
7.3.1.1 History
Since the thyroid pathology may have genetic determinism, the history is one of the first steps in the evaluation of the patient.
Nowadays, a special attention is given to patient history, due to severe increasing of nodular goiter, in order to have or not an aggressive approach of the nodule.
The history will bring information related to:
Age (elder patients need special attention firstly because of the evident difference in cancer evolution, age being an important staging factor)
Gender (even if female patients are more frequently affected by thyroid diseases, the male ones are related to a more aggressive evolution)
Exposure to radiation (in this case, a nodular goiter must be very carefully investigated for the exclusion of cancer)
Signs/symptoms of hyper-/hypothyroidism
Rapid change in volume or size of the nodule
The pain may indicate hemorrhage into nodule.
The lack of pain may indicate a malignant pathology.
The history may report the association of thyroid pathology with other syndromes:
Gardner’s syndrome (familial adenomatous polyposis)
Association with thyroid cancer.
Mostly in young women.
Thyroid cancer preceded the diagnosis of polyposis in 1/3 of cases.
Cowden syndrome (mucocutaneous hamartomas, keratosis, fibrocystic breast changes, and gastrointestinal polyps)
Association with thyroid cancer
Familial medullary thyroid carcinoma (MTC)
Familial MTC or multiple endocrine neoplasia (MEN II)
Family cases of other thyroid cancers
History elements suggestive for malignancy:
Rapid progressive enlargement
Hoarseness
Dysphagia
Dyspnea
Lymph nodes
7.3.1.2 Physical Examination
The situation of the thyroid gland in the anterior neck region gives a unique chance to this organ to be very easily inspected and to signalize the presence of diseases.
The thyroid gland can be seen easily by tilting the patient’s head backward. This position raises the thyroid up from the supraclavicular region and throws it into relief against the structures of the neck. Because the thyroid is fixed to the pretracheal fascia, it moves with the trachea when the patient is swallowing. This fact is very important in the differential diagnosis, with other structures that may appear in the anterior cervical area (e.g., adipose tissue). The large goiter may deviate the trachea, and this is the reason why it is mandatory to palpate it.
The palpation of the thyroid gland facing the patient and standing behind him gives the physician the necessary information regarding the structure of the gland and its relation with the surrounding tissues. The palpation of the lymph nodes situated on the neck should be carefully done, undergoing the known pathways of lymphatic drainage: lateral-cervical, supraclavicular, and submental.
The gland and the lymph nodes:
Inspection (enlargement, asymmetry, color, pulsation).
The patient drinks a glass of water so the physician can observe the swallowing (mobility of the gland, difficulties in swallowing, etc.).
Palpation.
Texture—soft/firm
Surface—smooth/seedy/lumpy
Volume—increased in different grades (diffuse, unilateral/nodular) and decreased/atrophic
Presence of regional pathologic lymph nodes
Spontaneous pain or pain at palpation
Mobility over the neck structures and with swallowing
The eyes and the face:
Presence of ophthalmopathy, grade, and evolution
Puffy face, facial, or general edema
Tongue edema and thyroid ectopy behind the tongue base
The general signs:
Tremor
Sweating
Diarrhea/constipation
Anxiety, depression, and lethargy
Heat/cold intolerance
Coarse skin
Weight loss/weight gain
Pretibial edema
Muscle weakness
Increased/reduced heart rate
Dry hair
All these signs and symptoms come to design a clinical presentation that may very seriously affect the patient status.
A complete clinical exam is able to suggest the diagnosis of the thyroid pathology, but the positive diagnosis of the type of thyroid disease is sometimes very difficult and energy consuming.
There are many evaluation guidelines continuously published, and many scientists have huge interest in the optimization of the procedures.
7.3.2 Laboratory Serum Testing
7.3.2.1 Thyroid-Stimulating Hormone
The first generation of TSH assays was based on radioimmunoassay (RIA) methodology, with limited functional sensitivity. Currently, most TSH testing is performed on automated immunoassay platforms employing non-isotopic technology. Serum TSH normally exhibits a diurnal variation with a peak between midnight and 4:00 a.m.; TSH testing is not usually influenced by the time of day of the blood draw.
Current guidelines worldwide recommend that serum TSH be used as the first-line test for detecting both overt and subclinical hypo- or hyperthyroidism in ambulatory patients with stable thyroid status and intact hypothalamic/pituitary function.
The current standards of care necessitate that laboratories use third-generation TSH assays, with the possibility of detecting serum TSH values lower than 0.1 mIU/L. This level of sensitivity is necessary for detecting differing degrees of TSH suppression.
In addition, targeting the degree of TSH suppression plays a critical role in the management of thyroid cancer.
In general, a normal reference range for TSH for adults is between 0.4 and 5.0 mIU/L, but values vary slightly among different types of analyzers.
TSH values in thyroid pathology may be:
Under lower limit establishing the diagnosis of clinical or subclinical hyperthyroidism
In normal range—euthyroid status (normal)
Over higher limit establishing the diagnosis of clinical or subclinical hypothyroidism
7.3.2.2 Thyroid Hormones (T4, T3, FT4, FT3)
Total thyroxine (T4). Most of the thyroxine (T4) in the blood is attached to a protein called thyroxine-binding globulin. Less than 1% of the T4 is unattached. A total T4 blood test measures both bound and free thyroxine. Free thyroxine affects tissue function in the body, but bound thyroxine does not. Normal range is between 57 and 148 nmol/L.
Free thyroxine (FT4). Free thyroxine (T4) can be measured directly (FT4) or calculated as the free thyroxine index (FTI). The FTI tells how much free T4 is present compared to the bound T4. The FTI can help tell if abnormal amounts of T4 are present because of abnormal amounts of thyroxine-binding globulin. Normal values of FT4 are 10–26 pmol/L.
Triiodothyronine (T3). Most of the T3 in the blood is attached to thyroxine-binding globulin. Less than 1% of the T3 is unattached. A T3 blood test measures both bound and free triiodothyronine. T3 has a greater effect on the way the body uses energy than T4 even though T3 is normally present in smaller amounts than T4. Normal values are 80–200 μg/L (1.2–3.1 nmol/L). For FT3, the normal values are 260–480 ng/L (3.4–6.8 pmol/L).
Thyroid hormone tests are done to find out what is causing an abnormal thyroid-stimulating hormone (TSH) test, to check how well treatment of thyroid disease is working, and for screening newborns, to find out if the thyroid gland function is normal.
In practice, measurement of free thyroxine and free triiodothyronine is a more reliable test of thyroid function than measurement of total hormone levels.
7.3.2.3 Antithyroid Peroxidase Antibodies/Anti-thyroperoxidase (Anti-TPO) Antibodies
Anti-TPO is a large, dimeric, membrane-associated, and globular glycoprotein that is expressed on the apical surface of thyrocytes.
Anti-TPO can be detected using complement fixation, agglutination test, or immunofluorescence on thyroid tissue sections, but concentrations are most commonly estimated using enzyme-linked immunosorbent assay or other sensitive and specific (manual or automated) immunoassays.
Anti-TPO prevalence is significantly higher in dietary iodine-sufficient countries like the USA and Japan as compared with iodine-deficient areas. The prevalence of anti-TPO is higher in women of all age groups and ethnicities and increases with age.
Anti-TPO measurement may serve as a useful prognostic indicator for future thyroid dysfunction. It may have an important clinical application to employ the presence of serum anti-TPO as a risk factor for developing thyroid dysfunction in patients receiving amiodarone, interferon alpha, interleukin-2, or lithium therapies.
Serial anti-TPO measurements are not recommended for monitoring treatment for autoimmune thyroid diseases. This is not surprising since the treatment of these disorders addresses the consequence (thyroid dysfunction) and not the cause (autoimmunity) of the disease.
Normal values are considered below 34 IU/mL.
7.3.2.4 Thyroglobulin
The tissue-specific origin of Tg has also led to its widespread use as a tumor marker for differentiated thyroid cancers (DTC).
Over the last 10 years, laboratories have adopted the more rapid, automated Tg immunometric assays (IMA) to replace the older isotopic radioimmunoassay (RIA). However, some laboratories still retain a Tg RIA because this methodology appears less prone to interferences from Tg autoantibodies (TgAb) and heterophilic antibodies (HAMA).
Unfortunately, serum Tg measurement is technically challenging. There are five methodologic problems that impair the clinical utility of the test:
Between-method biases
Insensitivity
Suboptimal inter-assay precision
“Hook” problems (IMA methods)
Tg autoantibody (TgAb) interference
For these and other reasons, current guidelines of treatment of DTC stress the critical importance of maintaining the same Tg method for serial monitoring of patients with DTC because a change in Tg method is very likely to produce misleading results. The American Thyroid Association and European Thyroid Association guidelines for managing patients with DTC state that an undetectable basal and recombinant human TSH (rhTSH)-stimulated Tg should be used as a parameter for the absence of disease. Specifically, a basal (unstimulated) Tg measurement below 0.1 μg/L predicts a negative rhTSH (recombinant human TSH) test (rhTSH-stimulated Tg below 2.0 μg/L) with a high degree of confidence.
Thyroglobulin values measured by immunometric methods have more pronounced variation than those determined by radioimmunology methods. This difference is probably due to the use of polyclonal antibodies with broad epitope specificity for radioimmunological determinations. This enables them to measure a wider range of abnormal tumor-derived thyroglobulin isoforms than immunometric assay methods that use monoclonal antibodies with limited epitope specificity.
Current guidelines do not routinely recommend preoperative serum Tg measurement.
Serum Tg measurements performed even as early as 6–8 weeks after surgery have been shown to be of important prognostic value. The serum Tg half-life approximates 3 days, and the acute injury-related Tg release resulting from the surgical procedure should largely resolve within the first 2 months.
The trend in serum Tg, measured over time and under constant TSH conditions, has proven to have a higher diagnostic sensitivity than using a fixed serum Tg cutoff value, especially when measured using a sensitive second-generation assay.
Because Tg protein is tissue specific, the detection of Tg in non-thyroidal tissues or fluids (such as pleural fluid) indicates the presence of metastatic thyroid cancer (Mazzaferri 2003). Struma ovary is the only (rare) condition in which the source of Tg in the circulation does not originate from the thyroid.
7.3.2.5 Antithyroglobulin Antibodies
Antithyroglobulin antibodies interfere with the accurate measurement of serum thyroglobulin. Serial antithyroglobulin antibody monitoring necessitates the use of the same method each time because assays vary in sensitivity, specificity, and absolute values. Current guidelines recommend that a sensitive TgAb immunoassay rather than an exogenous Tg recovery test be employed for this screening because a Tg recovery assessment has been shown to be an unreliable tool for detecting interfering TgAb.
There has been a growing recognition that serial TgAb measurements can serve as a surrogate tumor marker postoperatively. Supporting this view is the finding that patients who have serum TgAb detected at the time of initial surgery and who are rendered disease-free show a progressive decline in serum TgAb levels that become undetectable over a median period of 3 years. In contrast, patients with persistent/recurrent disease typically maintain detectable and often exhibit rising TgAb concentrations in association with tumor recurrence.
The use of a reference range derived from normal subjects is not recommended. Laboratories and manufacturers should determine and quote the minimum detection limit of their assays.
7.3.2.6 Calcitonin and Carcinoembryonic Antigen
Calcitonin is a marker for medullary thyroid cancer (MTC). Elevated serum calcitonin levels are highly suggestive for MTC; calcitonin levels may also be increased, although infrequently, in other clinical conditions such as C-cell hyperplasia, pulmonary and pancreatic neuroendocrine tumors, renal failure, and hypergastrinemia. Depending on the method used, smoking may either increase or decrease calcitonin concentration.
At present, the infusion of pentagastrin (0.5 μg/kg over 5 s), with determination of calcitonin levels at 0, 1, 2, 5, 10, and 15 min, appears to be the best test but is limited to countries where pentagastrin is available.
Basal calcitonin values are normally under (depending on the laboratory) 30 ng/L. Values of 30–100 after pentagastrin indicate hyperplasia, and values over 100 typically indicate the presence of cancer. Calcitonin should drop to levels of <30 ng/L if the tumor is completely removed surgically. It should be noted that excess production of calcitonin is not unique for medullary cancer but can occur with granulomatous diseases and other cancers. Patients with the syndrome should also be studied with parathyroid hormone and catecholamine assays in order to determine the presence of other components of the syndrome.
Calcium infusion, although less sensitive than pentagastrin, is a practical and attractive alternative.
Calcitonin and calcium/pentagastrin-stimulated calcitonin are used as tumor markers to screen relatives and to monitor patients who have been treated for MTC.
The C cells also produce carcinoembryonic antigen (CEA) in large amounts; it does not offer any obvious advantage and lacks the specificity of CT determinations. Tumor dedifferentiation is associated with a fall of CT and increasing CEA.
7.3.2.7 RET Oncogenes and Medullary Thyroid Cancer
Serum calcitonin measurement, which was once the mainstay in the diagnosis of familial MTC, has been replaced by sensitive polymerase chain reaction (PCR) assays for germline mutations in the RET proto-oncogene.
Studies on patients with MEN I and MEN II indicated linkage to chromosomes 11 and 10, respectively. Subsequent studies demonstrated that the RET oncogene is present at 10q11.2. RET is a cell membrane receptor of the growth factor family, with tyrosine kinase function. In up to 97% of patients with MEN IIA, mutations are found in codons 609, 611, 618, 620, and 630 in exons 10 and 11. These all involve substitutions of other amino acids for cysteine and are thought to cause activation of the gene by aberrant disulphide bonding causing dimerization. Similar changes are seen in familial MTC.
7.3.2.8 TSH Receptor Autoantibodies (TRab)
Circulating TSH receptor subunits have been implicated as a potential antigen in the autoimmune disease process.
TRab tests are useful in the differential diagnosis of various causes of hyperthyroidism but are particularly relevant in predicting fetal and neonatal thyroid dysfunction due to transplacental passage of maternal TRab from mothers with active or previously treated Graves’ hyperthyroidism.
A TRab measurement prior to radioiodine therapy may also be useful in predicting the risk for exacerbating Graves’ opthalmopathy (GO).
7.3.2.9 Mutations for KRAS, HRAS, NRAS, and BRAF and Translocations of PAX8/PPARγ and RET/PTC
Mutations in BRAF have been found in 40–45% of papillary thyroid cancer and also in anaplastic thyroid cancer (30–40%) and poorly differentiated tumors (20–40%). The vast majority (98%) of BRAF mutation are V600E (valine to glutamic acid).
The AKAP9-BRAF rearrangement is another mechanism of BRAF activation in thyroid cancers. This translocation, which fuses the first eight exons of the A-kinase anchor protein 9 (AKAP9) gene with the C-terminal region (exons 9–18) of BRAF, is found in up to 11% of tumors associated with radiation exposure but in less than 1% of sporadic tumors.
RAS mutations (HRAS, NRAS, and KRAS) are found in all thyroid carcinomas; RAS mutations are identified in 10–20% of papillary carcinomas, 40–50% of follicular carcinomas, and 20–40% of poorly differentiated and anaplastic carcinomas. HRAS mutations are also found in ~25% of sporadic medullary thyroid cancers. RAS point mutations are mutually exclusive with other thyroid mutations such as BRAF, RET/PTC, or TRK rearrangements in papillary thyroid cancers. In follicular carcinomas, RAS mutations are mutually exclusive with PAX8-PPARγ rearrangements.
Approximately 10–20% of sporadic papillary thyroid cancers (PTCs) harbor RET fusions. The prevalence of RET rearrangements is higher in patients irradiated and in pediatric populations. Multiple different RET rearrangements have been described in PTCs, but RET/PTC1, RET/PTC2, and RET/PTC3 account for the vast majority of cases.
Nowadays there are available software which combine specialized cytopathology with validated, proven genomic analysis to deliver greater understanding of a patient’s thyroid nodule diagnosis. These can help doctors identify benign nodules when cytopathology is indeterminate, thereby reducing the number of unnecessary thyroid surgeries, and also provide valuable additive information that may help guide decisions on the appropriate surgery.
7.3.3 Fine Needle Aspiration Biopsy
Fine needle aspiration biopsy has become the standard first-line test for diagnosis because it is safe, efficacious, and cost-effective and permits individualized therapy and monitoring. Fine needle aspiration biopsy (FNAB) is the most important diagnostic tool in evaluating thyroid nodules, and it should represent the first intervention.
Indication:
To make a diagnosis of a malignant thyroid nodule
To help select therapy for a thyroid nodule
To drain a cyst that may be causing pain
To inject a medication to shrink a recurrent cyst
Technique:
21–25-gauge needle (21–25G)
Multiple passes
Ideally from the periphery of the lesion
Re-aspirate after fluid drawn
Immediately smeared and fixed
Extend the patient’s neck slightly and palpate the nodule. Clean the skin with alcohol and place a 21- to 25-gauge needle on the end of a syringe.
There is also an available technique using 21G 10 cm modified Menghini-type needle for cytologic and histologic biopsy sampling. The needle has a Menghini-type cannula and trocar point stylet, which is combined with a 10 mL syringe. By the help of a spring mechanism, the syringe plunger, which is connected to the stylet, can be charged and released automatically. After the punction, the material is smeared, and the slide is fixed in alcohol for Papanicolaou and hematoxylin-eosin staining. Some slides can be air-dried and stained with Romanowsky stain (Diff-Quick).
Successful diagnosis by the cytologist depends on accurate sampling of the nodule and specimen cellularity. Ultrasonographic guidance can help to increase the accuracy of FNAB.
The combination of cytology and serum Tg determination in the aspirate fluid increases sensitivity. In cases of lymph node metastases, the Tg concentration in the aspirate fluid is often elevated (>10 ng/mL), and concentrations above this level are highly suspicious. A Tg concentration in the aspirate fluid between 1 and 10 ng/mL is moderately suspicious for malignancy, and comparison of the Tg measurement in the aspirate fluid and the serum should be considered in these patients.
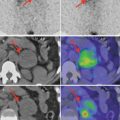
The possible results from FNAB are presented in Fig. 7.4:
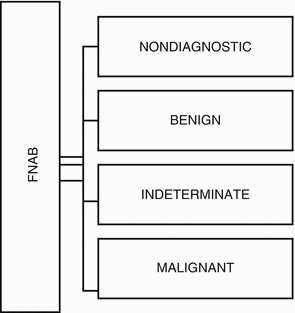

Fig. 7.4
FNAB cytology, according to the Bethesda System for Thyroid Cytology Classification
Bethesda System for Reporting Thyroid Cytopathology (Ali and Cibas 2010)
- I.
Nondiagnostic or unsatisfactory
Cyst fluid only
Virtually acellular specimen
Others (obscuring blood, clotting artifact, etc.)
- II.
Benign
Consistent with a benign follicular nodule (includes adenomatoid nodule, colloid nodule, etc.)
Consistent with lymphocytic (Hashimoto) thyroiditis in the proper clinical context
Consistent with granulomatous (subacute) thyroiditis
Other
- III.
Atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS)
- IV.
Follicular neoplasm (FN) or suspicious for a follicular neoplasm (SFN)
Specify if Hurthle cell (oncocytic) type
- V.
Suspicious for malignancy
Suspicious for papillary carcinoma
Suspicious for medullary carcinoma
Suspicious for metastatic carcinoma
Suspicious for lymphoma
Other
- VI.
Malignant
Papillary thyroid carcinoma
Poorly differentiated carcinoma
Medullary thyroid carcinoma
Undifferentiated (anaplastic) carcinoma
Squamous cell carcinoma
Carcinoma with mixed features (specify)
Metastatic carcinoma
Non-Hodgkin lymphoma
Other
Another classification for FNAB results (Collins 2015), frequently used in clinical practice, is the British system.
Thy 1—Nondiagnostic and insufficient sample. Cyst containing colloid or histiocytes only, in the absence of epithelial cells. The following act: to repeat FNAB. Ultrasound guidance may help. If cyst aspirated to dryness with no residual swelling, clinical/ultrasound follow-up alone may be sufficient.
Thy 2—Benign and nonneoplastic. Cyst containing benign epithelial cells.
Thy 3—Follicular or Hurthle cell lesion/suspected follicular or Hurthle neoplasm. Repeat FNAC in 3–6 months. Two nonneoplastic results 3–6 months apart should exclude neoplasia.
Thy 4—Suspicious of malignancy.
Thy 5—Diagnostic of malignancy.
The BTA updated in 2014 the terminology and introduced some subcategories. The Royal College of Pathologists publication should be followed for the terminology and is summarized below.
Thy1—nondiagnostic. This will include samples reflecting poor operator or preparation technique such as insufficient epithelial cells (the target is six groups each of at least ten well-visualized follicular epithelial cells) or only poorly preserved cells, as well as those reflecting the lesion such as cysts. The suffix Thy1c is used for cyst fluid samples with insufficient colloid and epithelial cells.
Thy2—nonneoplastic. These samples are adequately cellular and suggest a nonneoplastic lesion such as normal thyroid tissue, a colloid nodule, or thyroiditis. Cyst samples containing abundant colloid, even if less that the target number of epithelial cells, can be categorized as Thy2c.
Thy3—neoplasm possible. This is subdivided into:
Thy3a—when there are atypical features present but not enough to place into any of the other categories. The cytological interpretation should be clearly stated in the report and may include situations such as inability to exclude a follicular neoplasm or papillary carcinoma. Many Thy3a cases reflect suboptimal specimens and can be reallocated on repeat cytology.
Thy3f—when a follicular neoplasm is suspected. The histological possibilities then include a hyperplastic nodule, follicular adenoma, or follicular carcinoma. These cannot be distinguished on cytology alone, and a histology sample (lobectomy) will be required for diagnosis. Follicular variant of papillary thyroid carcinoma will also sometimes fall into this category.
Thy4—suspicious of malignancy but definite diagnosis of malignancy is not possible. The type of malignancy suspected should be stated in the report and is usually papillary carcinoma.
Thy5—diagnostic of malignancy. The type of malignancy should be stated, for example, papillary, medullary, or anaplastic thyroid carcinoma, lymphoma, or metastatic disease.
The likelihood of a malignancy on subsequent histology increases with increasing Thy category. There is also a false-negative rate for benign (Thy2, Thy1) cytology results, a fact that needs to be known.
Results of FNAB determine the next step in managing the thyroid nodule. Patients whose findings are nondiagnostic despite repeated biopsy can undergo surgery for lobectomy for tissue diagnosis, or they can be clinically monitored.
In these circumstances, radioiodine/technetium scans can be useful for determining the functional status of the nodule, as most hyperfunctioning nodules are benign (Piccardo et al. 2016).
Malignant diagnostics require surgical intervention. Papillary thyroid carcinoma and MTC are often positively identified on the basis of FNAB results alone. In patients with these carcinomas, definitive surgical planning can be undertaken at the outset. However, it is nearly impossible to distinguish a follicular adenoma from a follicular carcinoma on the basis of FNAB findings. Patients with follicular neoplasm, as determined with FNAB results, should undergo surgery for thyroid lobectomy for tissue diagnosis. These patients require complete thyroidectomy if a malignancy is discovered on review of the pathology.
Complications
The FNAB complications are few and generally minor. The most common complications are minor hematoma, ecchymosis, and local discomfort. Clinically significant hematoma and swelling are exceedingly rare. Inadvertent puncture of the trachea, carotid artery, or jugular vein usually does not cause clinically significant problems and is managed with the application of local pressure.
The results of FNAB will decide the following diagnostic and/or therapeutic strategies. Considering the recommendation of the latest American Thyroid Association thyroid nodule and differentiated thyroid carcinoma guidelines (ATA 2015), the algorithm for evaluation and management of patients with thyroid nodules based on US pattern and FNA cytology is presented in Fig. 7.5.
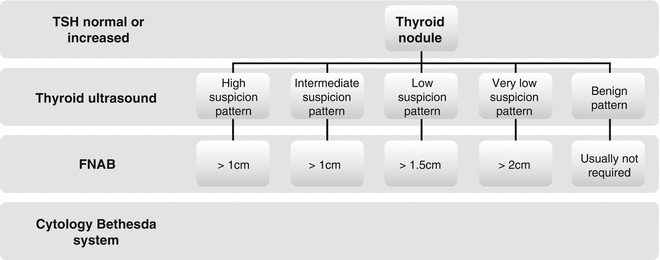

Fig. 7.5
The algorithm for FNAB evaluation and management of patients with thyroid nodules based on US patterns (ATA 2015)
7.3.4 Imagistic Diagnostic
7.3.4.1 Plain Films
Not routinely ordered, but these films may show:
Tracheal deviation
Pulmonary metastasis
Calcifications (suggests papillary or medullary carcinoma)
7.3.4.2 Ultrasonography
Ultrasonography (US) is the simplest, noninvasive test for the evaluation of thyroid gland. US should evaluate the following: thyroid parenchyma (homogeneous or heterogeneous) and gland size; size, location, and sonographic characteristics of any nodule(s); and the presence or absence of any suspicious cervical lymph nodes in the central or lateral compartments. The US report should convey nodule size (in three dimensions) and location (e.g., right upper lobe) and a description of the nodule’s sonographic features including consistency (solid, cystic proportion, or spongiform), echogenicity, margins, presence and type of calcifications, and shape if taller than wide, and vascularity. The pattern of sonographic features associated with a nodule confers a risk of malignancy and, combined with nodule size, guides FNA decision-making (ATA 2015).
Thyroid ultrasound should be used for:
Screening for thyroid presence in children
Differential diagnostic of cystic nodule vs solid nodule
Localization for FNAB or injection
Serial exam of nodule size
2–3 mm lower end of resolution
May distinguish solitary nodule from multinodular goiter
Nodule risk evaluation, mainly in Doppler mode or elastography
Findings suggesting for malignancy:
Presence of halo.
Irregular border.
Presence of cystic components.
Presence of calcifications.
Heterogeneous echo pattern.
Extrathyroidal extension.
Enlarged lymph nodes with specific patterns.
No findings are definitive.
Anarchic vascularity.
Rigidity at elastography.
The risk of malignancy is strongly correlated with some US patterns; many authors worldwide are being challenged to find the most appropriate criterion to be used in the differential diagnosis of benign/malignant thyroid nodules.
A schematic overview of some of the most important US parameters is presented in Fig. 7.6. The author’s experience notes that the character of the blood flow distribution within the nodule is an important factor that should be considered and carefully analyzed.
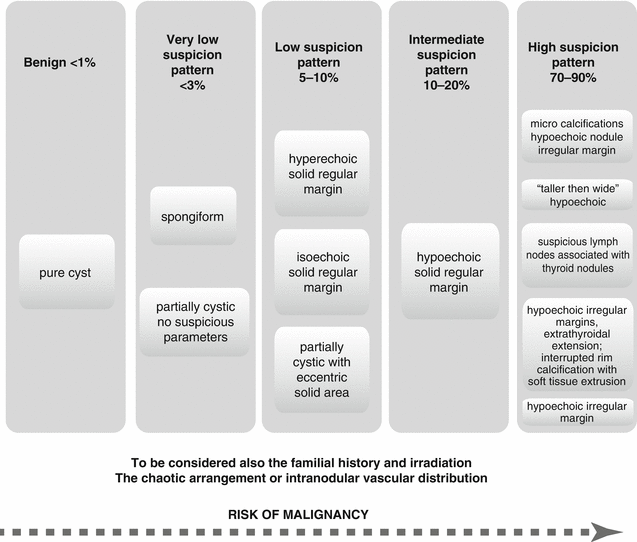

Fig. 7.6
The risk of malignancy of thyroid nodules based on US patterns (ATA 2015)
The intense vascularity inside a thyroid nodule is more likely to add negative prognostic than a peripheral distribution, and even if as a single factor, it is not considered the most powerful criterion for the diagnosis of malignancy.
The results of the multicenter studies published by specialists in the latest years summarize the most significant parameters to be linked to malignancy as the following ones:
Marked hypoechogenicity
Microcalcifications
“Taller-than-wide” shape in transverse view
Infiltrative, irregular margins
Abnormal cervical lymph nodes
Considering the mentioned features, in order to have a common reporting system about the risk of malignancy in thyroid nodules, ATA proposed the TIRADS system, which is presented in Table 7.1
Table 7.1
ATA 2015 TIRADS
Pathology | TIRADS | Thyroid ultrasound |
|---|---|---|
Normal | 1 | Normal |
Benign | 2 | Typical thyroiditis |
Pure cyst | ||
Spongiform nodule | ||
Isolated macrocalcification | ||
Most probable benign | 3 | Isoechogenic/hyperechogenic |
None of the high suspicious aspects | ||
Low suspicious malignant | 4A | Moderately hypoechogenic |
None of the high suspicious aspects | ||
Highly suspicious malignant | 4B | One or two signs of high suspicious aspects and NO adenopathy |
Highly suspicious malignant | 5 | More than three signs of high suspicious aspects and/or adenopathy |
The role of modern techniques adjuvants to gray scale or Doppler ultrasound is controversial. An example is ultrasound elastography (USE) which is a measurement of tissue stiffness; initially it was considered to be an interesting additional tool in the diagnosis of malignancy in the thyroid (Bojunga et al. 2010). More recently, larger trials have reported substantially different results and lack of clear recommendation, mainly due to differences between the experience of the operators. Moon et al. (2016) showed that the performance of USE was inferior to that of gray-scale US assessment. The largest prospective study of 706 patients with 912 thyroid nodules was recently published by Azizi et al. (2013). In this study, the positive predictive value (PPV) of USE was only 36%, comparable to that of microcalcifications. Thus, at present, USE cannot be widely applied to all thyroid nodules in a similar fashion to gray-scale or Doppler US examination (Koh et al. 2016; Russ et al. 2013).
7.3.4.3 Computed Tomography Scanning, Magnetic Resonance Imaging, and Positron Emission Tomography Scanning
Computed tomography (CT) scanning or magnetic resonance imaging (MRI) is generally not cost-effective in the initial evaluation of the thyroid gland. Such studies may be useful in the assessment of thyroid masses that are largely substernal or in the assessment of the extension of a known thyroid tumor.
CT and MRI are indicated if there is presence of a fixed thyroid mass or of patients with hemoptysis, indicating potential involvement of surrounding structures. Other important indications include cervical lymph adenopathy or when limits of the goiter cannot be clinically determined. CT or MRI can demonstrate involvement of the larynx, pharynx, trachea, esophagus, or major blood vessels. It is important to avoid iodine contrast media in CT scan to ensure subsequent radioiodine treatment uptake. This difficulty may be overcome by requesting for gadolinium-enhanced MRI scan.
Positron emission tomography (PET) scanning with 18F-fluorodeoxyglucose is not an investigational tool with role in the primary evaluation of the thyroid nodule. Since the first edition of this book, the last 5 years demonstrated a crucial role of PET/CT F18-FDG mainly in the management of differentiated thyroid carcinoma, with detectable Tg and negative I-131 whole-body scan (WBS). There will be a special part dedicated to this topic.
In the past, nuclear imaging studies of the thyroid, often combined with ultrasonography, were routinely performed in initial assessment of thyroid nodules. Because only 10% of solitary thyroid nodules are hot and because 90% of cold nodules are not malignant, nuclear imaging with or without ultrasonography typically offers a low yield of cancer diagnosis in surgical specimens when their results are used as the main guides for referral to a surgeon.
Any incidental nodule detected on US should be assessed using the criteria discussed above. Incidental nodules detected on computed tomography (CT) can be more problematic; no CT feature reliably distinguishes benign from malignant lesions. The incidentaloma detected on PET/CT F18-FDG should be evaluated with special attention.
7.3.5 Nuclear Imaging Tests
7.3.5.1 Thyroid Scintigraphy with Tc-99m Pertechnetate (Tc-99m Pt) Gamma Camera
Radiopharmaceutical:
Technetium-99m pertechnetate (Tc-99m Pt)
Principle:
Tc-99m Pt is trapped from the bloodstream into the thyroid gland but without organification.
Technique:
Patient preparation: No special preparation needed.
No need for fasting
No need for thyroid hormone withdrawal
No significant influence caused by other drugs
No influence caused by recent procedures with iodine contrast substances
Attention to breastfeeding patients, children, and potential fetal exposure
Dose: 74–200–370 MBq/patient of Tc-99m Pt obtained from generator of Tc-99m in the department, respecting the quality control rules.
Dose calibration.
Injected I.V.
15–30 min are necessary for waiting, after injection.
Patient position: Supine, slight neck extension.
Gamma camera:
Daily calibration and valid quality control tests
Small-field rectangular/pinhole collimator
Low-energy high-resolution (LEHR) collimator
Incidence: Anterior-posterior (AP) over the patient’s neck, as close to the neck as possible, avoiding the influence of resolution by an inadequate distance. At 10 cm from the neck, this distance gives a magnification factor of 2–2.5.
Acquisition:
Static acquisition.
Selected energy 140 keV.
Window 15–20%.
128 × 128 matrix; 64 × 64 might be used also in special situations.
Minimum 250,000 counts/image.
Mark the suprasternal notch, with a point source of Tc-99m Pt or Cobalt-57; this mark will be registered on the images. This is important mainly in case of ectopy of the thyroid gland or in case of important enlargement and retrosternal position.
Lateral or oblique-lateral images may be also obtained.
Any palpable nodule should be marked on the images, during the acquisition, giving to the physician the facility to position the nodule.
Processing: There is no need of special PC programs; an additional may be used in the analysis of counts in different regions of interest (ROI); image interpretation criteria are detailed below.
Clinical applications:
The diagnosis of hyperthyroidism in:
Hyperfunctional nodular goiter
Autonomously hyperfunctioning adenoma (“hot” nodule)
Hyperfunctional diffuse goiter (Basedow-Graves’ disease)
The diagnosis of hypothyroidism in:
Primary congenital hypothyroidism (myxedema)
Secondary hypothyroidism
Thyroid cancer (cold nodule)
de Quervain thyroiditis and chronic thyroiditis (Hashimoto’s disease)
Thyroid cysts and other causes of nodules
Thyroid anatomical variants: Retrosternal goiter, thyroid sublingual, left or right lateral-cervical thyroid, accessory lobe (Lalouette’s pyramid), and agenesis of a lobe or of the entire gland
Necessary additional examinations:
Clinical examination; remember to discuss and to examine the patient before the injection, in order to limit the hazardous radiation exposure.
Thyroid ultrasound.
Serologic tests: TSH, FT4, FT3, anti-TPO antibodies, anti-Tg, calcitonin, thyroglobulin, TRab, etc.
Fine needle aspiration biopsy (FNAB).
Comments:
The place of scintigraphy in the assessment of thyroid diseases, especially of thyroid nodules, is well established by the national and international algorithms of diagnostic and treatment of thyroid nodules and availability of tracers (Velzen et al. 2005).
Reports:
The reports respect the general format of the department with all the identification data of the patient, the institution, and the physician. The report will include the technical data related to the radiopharmaceutical, the used dose, the type of gamma camera, and the acquisition data. The report will note the estimation of the absorbed dose due to this examination. These data are available in predefined forms calculated for the majority of tracers and investigations.
The report will include a description of the:
Shape
Position
Size
Contour
Presence or absence of nodules in the thyroid area; the distribution of the radiotracer in the entire gland, in each lobe and in the nodules
Presence of any special findings on scintigraphy
The normal image of thyroid scan with Tc-99m Pt is shown below (Figs. 7.7 and 7.8)
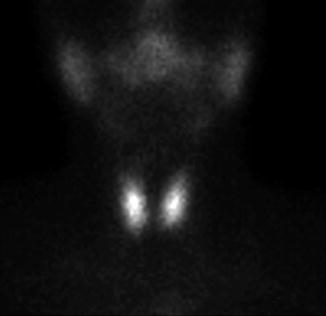
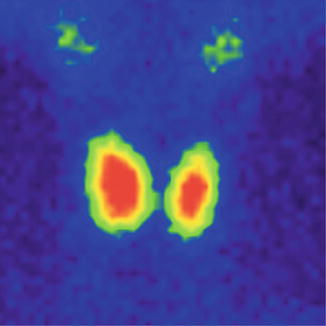

Fig. 7.7
Normal thyroid scan with Tc-99m Pt. Color scale—gray

Fig. 7.8
Normal thyroid scan with Tc-99m Pt. Color scale—Sopha Rainbow
The thyroid scan will show:
The typical “butterfly” shape.
The normal dimensions according to laboratory standard (these must be seated in relation with the area particularities and respecting the age variations).
The position in the anterior neck area, above the sternal notch, in the region of the thyroid cartilage.
The regular contour, without any interruption or mismatches.
The homogenous distribution of the radiotracer; with intense color in the middle of each lobe related to the thickness of the thyroid tissue; the margins will have a less intense color, due to the decreasing quantity of the thyroid tissue. The image must present the color scale used in processing, so the physician will be able to correlate the percent of activity with the color.
The absence of nodules with different metabolic activities.
There is a normal visualization on the scan of the salivary glands. Physiologically the right lobe may be larger than the left one.
We need to give special attention to technical details: the matrix, the color adjustment, and the image zoom; these may significantly modify the final presentation of the thyroid’s images.
In the next two images, there is an example of important differences in the thyroid’s images due to different details of acquisition:
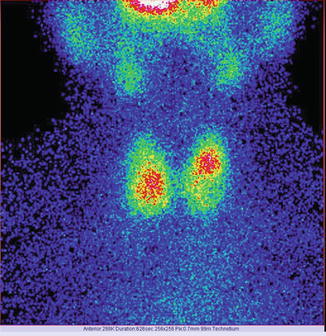
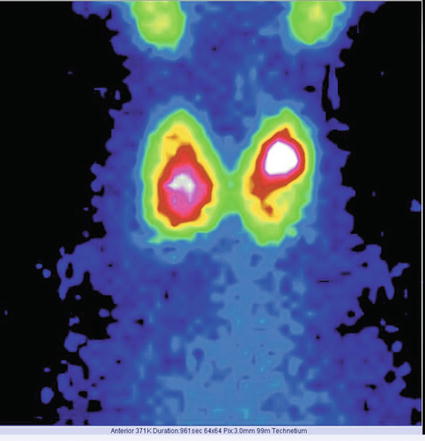

Fig. 7.9
Thyroid scan Tc-99m Pt obtained with a matrix of 256 × 256

Fig. 7.10
Thyroid scan Tc-99m Pt obtained with a matrix of 64 × 64
Case 1: Autonomously Functioning Thyroid Nodule
History:
A 57-year-old male, with previous history of irradiation of the neck for a larynx neoplasm, 8 years before, returns for evaluation.
Clinical examination:
Symptoms: tremor, weight loss of 12 kg in 2 months, sweating, insomnia, and tachycardia.
The neck examination revealed a tumor mass in the anterior neck area, not very firm, with elastic structure, ascending when the patient is swallowing, developed quickly in the last 5 weeks. No clinical evidence of lymph nodes. The heart rate was 120/min. No signs of exophthalmia.
Examinations:
The ultrasound revealed a solid, hypoechogenic nodule involving almost the entire left lobe of the thyroid. The nodule has regular contour and intense vascularity. No pathologic lymph nodes.
TSH—0.15 mIU/L (N.V. 0.4–4.5 mIU/L), low
Findings:
The scintigraphy with Tc-99m Pt showed an intense uptake of the radiotracer in the entire left lobe of the thyroid (“hot” nodule), corresponding to the ultrasound finding.
The rest of the gland did not trap the tracer, due to the suppression of the tissue, by the dominant nodule; the rest of the gland did not appear on the image (Fig. 7.11).
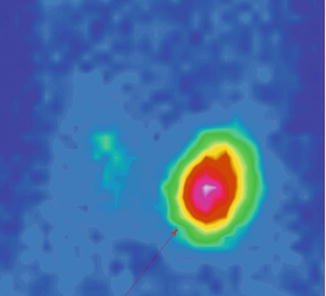

Fig. 7.11
Left thyroid lobe completely “hot” nodule
Conclusion:
Autonomously hyperfunctioning left thyroid nodule (toxic adenoma)
Key Points
In the context of previous neck neoplasm and history of irradiation, it was mandatory to make the differential diagnosis of autonomously functioning adenoma with larynx neoplasm recurrence or primitive thyroid cancer.
The frequency of thyroid cancer appearing in autonomous adenoma is worldwide communicated as being very low, but it does exist.
It is important to keep in mind that even in this situation, to rule out the cancer is essential. Careful posttherapy follow-up of the patient and of the structure must be done. Increasing the volume or special patterns in ultrasound must indicate the FNAB.
Several cases of autonomously hyperfunctioning “hot” nodule of differentiated thyroid carcinoma were published in the literature.
The initial FNAB in this particular case is not recommended.
Similar cases: Figs. 7.12, 7.13, and 7.14:
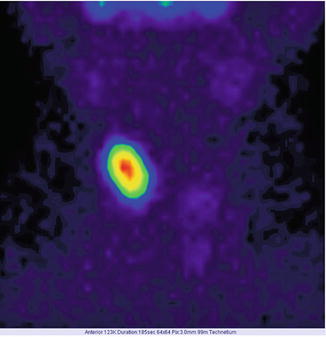
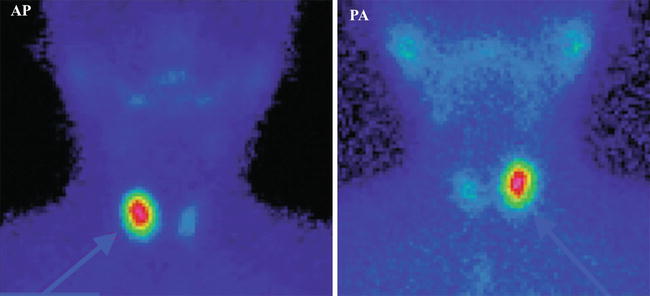
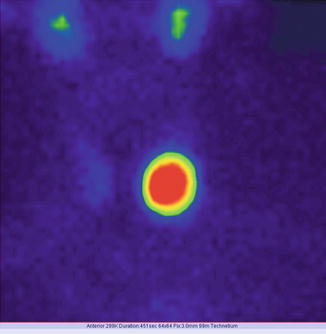

Fig. 7.12
“Hot” nodule in the upper pole of the right thyroid lobe; suppression of the surrounding thyroid tissue

Fig. 7.13
“Hot” nodule in the right lobe—toxic adenoma. Incidence AP and PA

Fig. 7.14
“Hot” nodule involving the entire left thyroid lobe; suppression of the surrounding thyroid tissue
Case 2: Papillary Thyroid Carcinoma
History:
A 36-year-old female, with family history of thyroid cancer (father died with papillary thyroid carcinoma), is referred.
Clinical examination:
Insomnia and anxiety; swallowing difficulties.
The neck examination revealed enlargement of the thyroid gland, with a tumor mass of about 2.5 cm in the maximum diameter, in the central and left anterior neck area, with firm structure, ascending when the patient is swallowing.
The cervical tumor developed slowly in the last 3 years. No medication was administered for this pathology during this time. No clinical evidence of lymph nodes. No other signs or symptoms to be mentioned.
Examinations:
The ultrasound revealed a solid, hypoechogenic nodule 1.4/2.2 cm in the lower part of the left thyroid lobe, extended to the isthmus, regular contour but anarchic vascularity mainly in the periphery of the lobe. There were no calcifications.
The presence of another hypoechogenic nodule in the 1/3 medium to the lower part of the left lobe has been observed. This nodule is in the strict vicinity of the thyroid capsule and measures 1.3/1.9 cm. The nodule had regular contour and intense vascularity, mainly in the interior of the nodule. The right lobe was homogenous. No pathologic lymph nodes (Fig. 7.15).
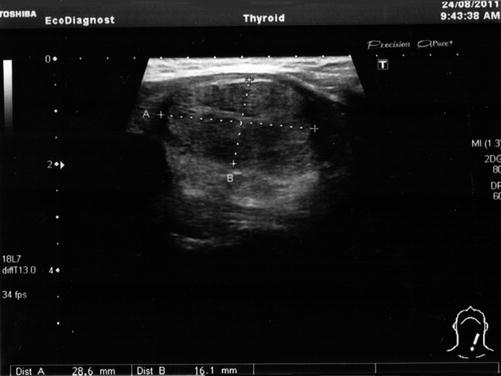

Fig. 7.15
Thyroid ultrasound (With the courtesy of Dr. H. Branda—Ecodiagnost Cluj-Napoca)
TSH—2.31 mIU/L (N.V. 0.4–4.5 mIU/L), normal.
Anti-TPO < 34 (N.V. <34 mIU/L), normal.
FNAB previously performed 8 months ago—benign cytology (Thy 2)
Findings:
The scintigraphy with Tc-99m Pt showed a diffuse inhomogeneity of the uptake in the entire left thyroid lobe and isthmus.
There are two different metabolic nodular structures: the first is situated in the upper position, at the junction of medium to the lower third of the lobe, with less uptake of radiotracer, hypoactivity, and in some area even lack of the tracer uptake (“cold” nodule); the second is situated in the inferior part extended to the isthmus, with metabolic activity almost normal, corresponding to the surrounding thyroid tissue.
The rest of the gland has a normal tracer distribution (Fig. 7.16).

Fig. 7.16
Thyroid scintigraphy Tc-99m Pt, AP incidence, showing the left thyroid lobe with multiple macronodules
Conclusion:
Multiple left macronodular goiter. Family history of thyroid cancer. FNAB recommended (Fig. 7.17). The results of FNAB will establish the type of surgical intervention (left lobectomy or total thyroidectomy).
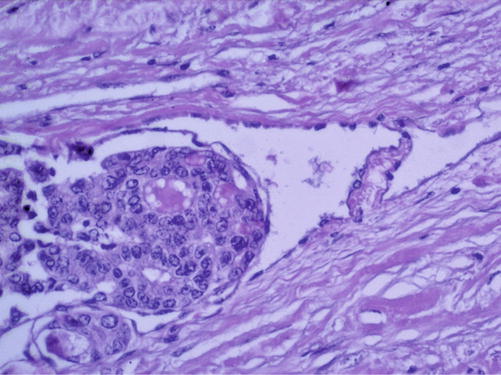

Fig. 7.17
Papillary thyroid carcinoma. Hematoxylin-eosin staining ×200 (With the courtesy of the Pathology Department of the Institute of Oncology Prof. Dr. Ion Chiricuţă Cluj-Napoca)
Similar cases: Figs. 7.18, 7.19, 7.20, and 7.21
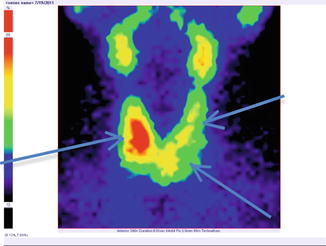
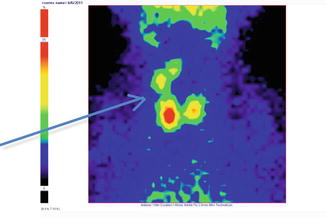
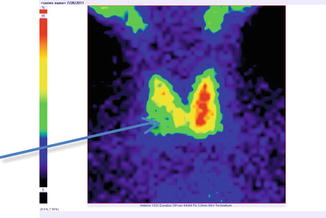
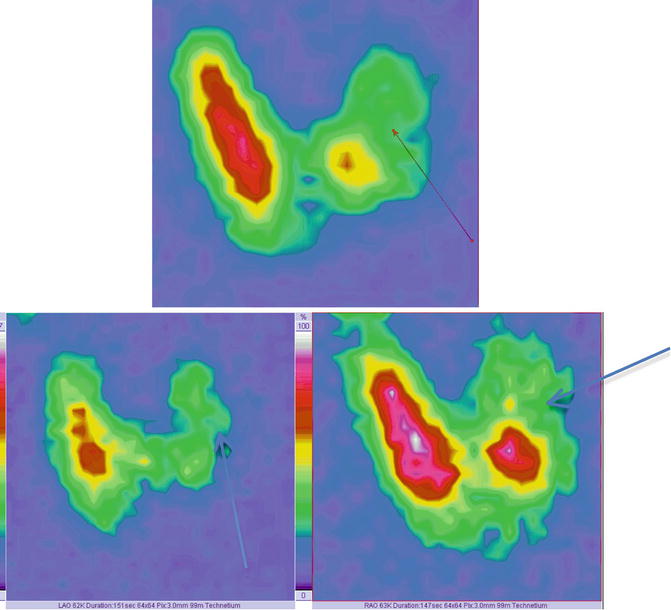

Fig. 7.18
Thyroid scintigraphy Tc-99m Pt, AP incidence, showing “cold” nodule in the lower pole of the left thyroid lobe extended to the isthmus. Similar structures appear in the superior left lobe and inferior pole of the right lobe

Fig. 7.19
Thyroid scintigraphy Tc-99m Pt, AP incidence, showing “cold” nodule in the upper pole of the right thyroid lobe

Fig. 7.20
Thyroid scintigraphy Tc-99m Pt, AP incidence, showing “cold” nodule in the lower pole of the right thyroid lobe

Fig. 7.21
Thyroid scintigraphy Tc-99m Pt showing left “cold” nodule. Images in AP incidence (top of image); left anterior oblique (LAO) and right anterior oblique (RAO)—bottom of the image
The oblique incidence permits a more accurate visibility of the nodule, mainly situated in the posterior part of the left lobe.
These supplementary incidences may be useful especially in differential diagnosis of parathyroid adenoma or thyroid nodule.
Key Points
The family history of thyroid cancer makes from this case an example of the special attention to be given to the evaluation of thyroid nodules appearing in such families.
The evolution in time, the significant growth of both nodules in a short period, marked an alert and imposed an aggressive approach.
The ultrasound showed the two nodular structures, both of them larger than 1 cm in the maximum diameter.
The result of previous FNAB was benign, but the physician aspirated only one nodule from the lower part, without knowing the difference between the metabolic behaviors of the two left nodular structures.
The second FNAB was targeted to the “cold” nodule as is shown in the scintigraphy, and the result was highly suspicious for papillary thyroid carcinoma.
The patient was referred to surgery and underwent total thyroidectomy with central pretracheal lymph node removal; the final histology report confirmed the papillary thyroid cancer, invasive in the adipose tissue near the thyroid gland.
The presence of a thyroid nodule, in strict relation with the thyroid capsule, needs special attention, with the intention to rule out the cancer; penetrating the capsule, the tumor cells will be invasive in the surrounding soft tissues and will change the patient’s group of risk.
Case 3: Retrosternal Nodular Goiter
History:
A 29-year-old female, without history of risk (no irradiation, no family history of thyroid cancer or other cancers), is referred.
Clinical examination:
Swallowing difficulties, mainly at physical efforts.
Breathing distress related to emotional situations, efforts, and high environmental temperature.
The neck examination does not reveal anything pathologic. No enlargement of the thyroid gland and no tumor in the neck area; no changes while patient has deglutition.
No clinical evidence of lymph nodes. No other signs or symptoms to be mentioned.
Examinations:
The ultrasound revealed that the thyroid gland had no nodules. It had regular contour and normal vascularity. No pathologic lymph nodes. It had an entirely homogenous appearance.
TSH—1.7 mIU/L (N.V. 0.4–4.5 mIU/L), normal.
Anti-TPO < 34 (N.V. <34 mIU/L), normal.
FT4—16.4 pmol/L (N.V. 12–22 pmol/L), normal.
Findings:
The scintigraphy with Tc-99m Pt showed a diffuse enlargement of the thyroid gland. The lower pole of the left lobe and especially the isthmus were significantly larger. These parts were descended in the upper mediastinum.
The tracer was distributed highly none homogenously in the mentioned area, with some zones of lack of the tracer’s uptake.
The rest of the gland has a normal tracer distribution (Fig. 7.22).
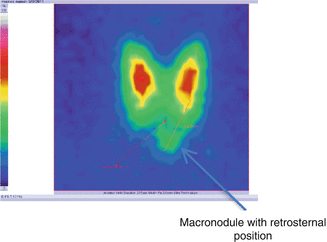

Fig. 7.22
Thyroid scintigraphy with Tc-99m Pt, AP incidence, which reveals nodular goiter with retrosternal position
Conclusion:
Macronodular goiter with retrosternal position. Patient underwent surgical removal (Fig. 7.23).
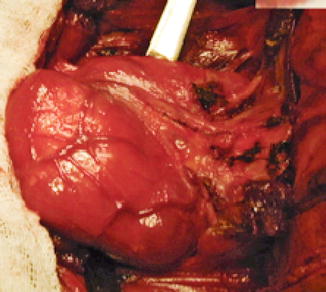

Fig. 7.23
Left thyroid lobe. Surgical specimen (With the courtesy of Prof. Dr. A. Irimie-Institute of Oncology “Prof. Dr. I. Chiricuţă” Cluj-Napoca)
Key Points
The thyroid ultrasound is not able to detect the thyroid tissue beyond the sternal notch.
In the situation of a goiter having a retrosternal position, the radiography may detect trachea deviation or compression.
Scintigraphy is able to confirm the thyroid origin of the mediastinum mass.
Tumors may be developed frequently, with an insidious evolution.
Case 4: Remnant Thyroid Tissue Postlobectomy
History:
A 42-year-old female with total right lobectomy 2 years before is referred to the department. Nodular goiter histology revealed no malignant suspicion.
Clinical examination:
No complaints; routine check of the thyroid function after the partial thyroidectomy. The neck examination does not reveal anything pathologic.
No enlargement of thyroid gland, no tumor in the neck area; no changes while patient is swallowing.
No clinical evidence of lymph nodes. No other signs or symptoms to be mentioned.
Examinations:
The ultrasound revealed that the remnant left thyroid gland had no nodules. It had regular contour and normal vascularity.
No pathologic lymph nodes. It had an entirely homogenous appearance.
In the right part of thyroid bed, an irregular structure of 1/1.2 cm, without vascularity, intensely hypoechogenic.
TSH—1.1 mIU/L (N.V. 0.4–4.5 mIU/L), normal.
Anti-TPO < 34 (N.V. <34 mIU/L), normal.
FT4—13.2 pmol/L (N.V. 12–22 pmol/L), normal.
Hormone values obtained under replacement dose of levothyroxine 50 μg/daily
Findings:
The scintigraphy with Tc-99m Pt showed a discrete diffuse enlargement of the left thyroid lobe.
The tracer was distributed homogenously in the lobe, without any inefficient metabolic zones.
There is no tracer uptake in the right area; existence of a total right lobectomy (Fig. 7.24).
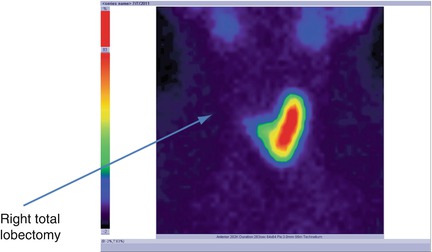

Fig. 7.24
Thyroid scintigraphy with Tc-99m Pt showing the absence of the radiotracer in the right area; right total lobectomy
Conclusion:
Right thyroid lobectomy. Normal left lobe. No nodular recurrence.
Key Points
After the surgical intervention in the thyroid area, thyroid ultrasound may detect abnormal structures, due to fibrosis, sutures, or hematoma.
Scintigraphy is able to confirm the presence or absence of metabolic active tissues.
Case 5: Subacute Thyroiditis
History:
A 22-year-old female is referred. The family history reveals mother with Hashimoto’s thyroiditis.
Clinical examination:
Symptoms: fine tremor of extremities, weight loss of 4 kg in 3 weeks, swelling, insomnia, and fatigue.
The neck examination revealed a discrete enlargement of the thyroid gland, with an elastic structure, ascending while patient has deglutition.
Multiple lymph nodes were palpable in the bilateral neck area, having the maximum diameter of 1.5 cm. The heart rate was 120/min. No signs of exophthalmia.
She presented important headache and minimal fever of 37.6 °C 3 weeks before, apparently with no connection with the thyroid disease.
Examinations:
The ultrasound revealed that the thyroid gland has regular contour and very intense diffuse vascularity. The entire gland has multiple micronodules in the structure, with no dominant nodule and no calcifications.
Pathologic multiple lymph nodes detected in the bilateral lateral-cervical area. Lymph nodes had inflammatory presentation on ultrasound.
TSH < 0.001 mIU/L (N.V. 0.4–4.5 mIU/L), very low, and undetectable
Anti-TPO > 600 mIU/L (N.V. <34 mIU/L), very increased
Anti-Tg—1280 kIU/L (N.V. <115 kIU/L), very increased
FT4 > 100 pmol/L (N.V. 12–22 pmol/L), very increased
EBR—12 mm/h, normal
TRab negative
Findings:
The scintigraphy with Tc-99m Pt showed a very low uptake, with inhomogeneity in the neck area.
There was a very low Tc-99m Pt uptake (0.1%), while the normal values are 0.7–1.4% (Fig. 7.25).
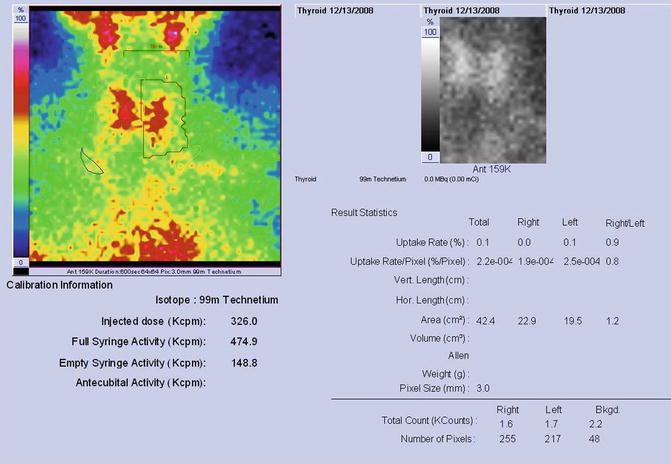

Fig. 7.25
Thyroid scintigraphy Tc-99m Pt and uptake values
Conclusion:
Subacute thyroiditis. There was a thyroid attack of an infectious mononucleosis disease (serologic confirmed in the next evaluation sequences).
There is also a possibility to have a complete blank scintigraphy, without any tracer uptake in the thyroid area (Fig. 7.26).

Fig. 7.26
Blank scintigraphy without any tracer uptake in the thyroid area
Key Points
The scintigraphy features in acute or subacute inflammatory pathology of the thyroid gland are specific; the total or near-total absence of the tracer’s uptake in the neck area is the characteristic presentation and may establish the diagnosis.
Also, it is important to have the uptake sequences, which will confirm the blockage of the tracer’s uptake (very low uptake values).
Regarding the correlation with the serum hormonal values, we must give special attention to the phase of the disease; in an early phase, due to tissue destruction, there may be a hyperfunctional context; the scintigraphy will set the differential diagnosis between thyroiditis and goiter with hyperthyroidism.
Sometimes the clinical evaluation is nearly normal, highly discordant with the serum hormonal values and nuclear metabolic tests.
Infectious diseases may affect the thyroid gland.
Case 6: Hashimoto’s Thyroiditis
History:
A 58-year-old female, menopause installed 11 years before. Weight of 11 kg gained in 8 months.
Clinical Examination:
She presented fatigue, insomnia, depression, diffuse muscle pain and periodic contracture, swallowing difficulties, “globus hystericus,” and postprandial pain in the right hypocondrium.
The neck examination does not reveal anything pathologic. No enlargement of thyroid gland, no tumor in the neck area.
No clinical evidence of lymph nodes. No other signs or symptoms to be mentioned.
Examinations:
The ultrasound revealed that the thyroid gland has a very important diffuse hypoechogenicity, with a multinodular structure in both lobes; micronodules (less than 1 cm in the largest diameter) with an important increase of vascularity. The thyroid had an irregular contour.
No pathologic lymph nodes.
TSH—14.1 mIU/L (N.V. 0.4–4.5 mIU/L), high
Anti-TPO > 600 (N.V. <34 mIU/L), high
FT4—7.31 pmol/L (N.V. 12–22 pmol/L), low
Hormone values obtained without any hormonal intake
Findings:
The scintigraphy with Tc-99m Pt showed a discrete diffuse enlargement of the entire gland.
The tracer was distributed without homogeneity in the gland, with some inefficient metabolic zones (Fig. 7.27).
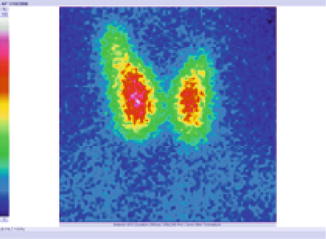

Fig. 7.27
Thyroid scintigraphy with Tc-99m Pt, AP incidence, which reveals inhomogeneous distribution of the tracer, suggestive for Hashimoto’s thyroiditis
Conclusion:
Hashimoto’s thyroiditis
Key Points
The presence of nodules in the area of a lymphatic thyroiditis suggests a very careful evaluation, with cytology description, in order to rule out the possible association with thyroid carcinoma.
Thyroid cancer is frequently developed in Hashimoto’s thyroiditis.
There is the possibility to develop hyperthyroidism associated to Hashimoto’s thyroiditis; this fact justifies the evaluation by nuclear tests, regarding a prospective radioiodine therapy.
Case 7: Thyroid Adenoma
History:
A 31-year-old male. No family history of irradiation or thyroid cancers.
Clinical examination:
Clinical evidence of left lateral-cervical lymph nodes, with elastic consistency and with a maximum diameter of 1.2 cm.
No complaints; routine check of thyroid gland during the evaluation of lymph nodes origin.
No enlargement of the thyroid gland and no tumor in the neck area; no changes while the patient has deglutition.
No other signs or symptoms to be mentioned.
Examinations:
The ultrasound revealed that the thyroid has regular contour and normal vascularity.
Pathologic enlargement of left lateral-cervical lymph nodes in the medial area of the neck, with characteristic pattern of inflammatory disease.
In the left part of the thyroid gland, we observed the presence of an intense hypoechogenic irregular structure of 2.41/1.2 cm, having an intense vascularity (Fig. 7.28).
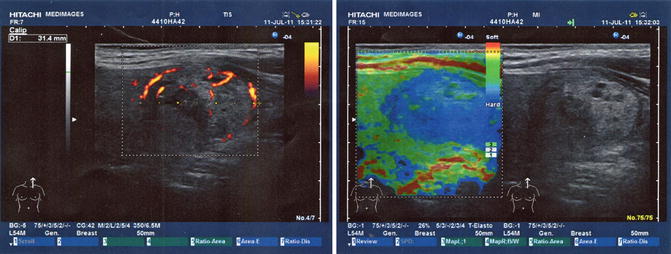

Fig. 7.28
Ultrasound elastography (With the courtesy of Dr. Angelica Chiorean—Medimages)
TSH—1.8 mIU/L (N.V. 0.4–4.5 mIU/L), normal.
Anti-TPO < 34 (N.V. <34 mIU/L), normal.
FT4—17.2 pmol/L (N.V. 12–22 pmol/L), normal.
Findings:
The scintigraphy with Tc-99m Pt showed the distribution of the “cold” nodule in the 1/3 part of the thyroid gland’s left lobe. This metabolic feature was very discordant with the thyroid ultrasound, which concluded the diagnosis of thyroid adenoma (Fig. 7.29).
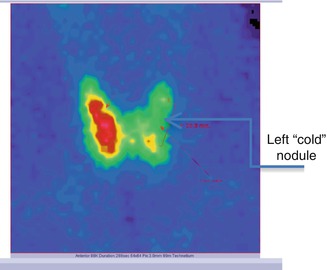

Fig. 7.29
Thyroid scintigraphy with Tc-99m Pt, AP incidence, showing the left thyroid lobe with “cold” nodule
Conclusion:
Left “cold” thyroid nodule. FNAB recommended.
In the case of thyroid adenomas, the uptake is normal, similar with that of the surrounding thyroid tissue (Fig. 7.30).
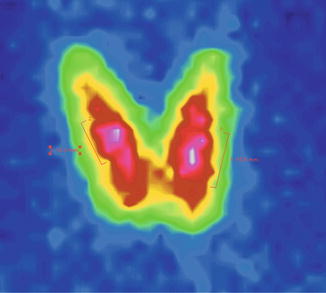

Fig. 7.30
Thyroid scintigraphy with Tc-99m Pt, AP incidence, with homogenous uptake nodules in both thyroid lobes. Thyroid adenomas
Key Points
Even if the ultrasound is very improbable to suggest cancer, do not hesitate to do the best in excluding cancer, especially if there are also other criteria of risk (familial history, irradiation, gender, age, or lymph nodes).
In these situations, scintigraphy may help to orient the physician to take an aggressive procedure and to perform FNAB.
Except the pathologist, nobody has the right to affirm or infirm with clear and maximum accuracy the diagnosis of cancer; based on images, there may be a high probability but not certitudes.
Case 8: Thyroid Cyst
History:
A 32-year-old female. No family history of irradiation or thyroid cancers. She recently experienced a very intense physical effort, during childbirth.
Clinical examination:
Clinical evidence of a cervical tumor in the right part of the anterior neck, developed in a few days after the birth travail. The tumor had an elastic consistency and a maximum diameter of 6.2 cm.
She was referred for the second opinion at 5 months after that moment, presenting an enlargement of the thyroid gland, discrete tension, and slight pain in the tumor area, ascending while patient has deglutition. Multiple right-side lateral-cervical lymph nodes detected at palpation. Previous FNAB with evacuation performed at the begging of the disease (4 months ago) was negative for malignant cells.
No other signs or symptoms to be mentioned, except for breastfeeding.
Examinations:
The ultrasound revealed an important enlargement of the right thyroid lobe, due to an intense hyperechogenic nodule, but with some interior areas of hypoechogenic structures with low vascularity. No calcification detected.
Multiple lymph nodes in the right lateral area of the neck, highly probable with inflammatory character.
The rest of the thyroid gland presented an irregular structure, but without delimited nodules. The conclusion was thyroid cyst with a possible hemorrhagic interior or organized hematoma.
TSH—2.91 mIU/L (N.V. 0.4–4.5 mIU/L), normal.
Anti-TPO < 34 (N.V. <34 mIU/L), normal.
FT4—12.2 pmol/L (N.V. 12–22 pmol/L), normal.
Findings:
The scintigraphy with Tc-99m Pt showed the distribution of a “cold” nodule in the lower part of the thyroid gland’s right lobe. Not all the nodule was completely metabolic inactive; there were some areas with tracer uptake, anarchically distributed in the nodule (Fig. 7.31).
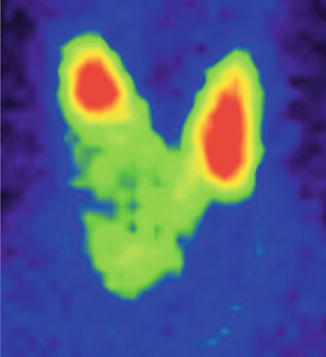

Fig. 7.31
Thyroid scintigraphy with Tc-99m Pt, AP incidence, showing a cyst of the right thyroid lobe with interior metabolic activity, due to papillary excrescences
In pure thyroid cyst, the nodule at scintigraphy appears complete metabolic inactive (Fig. 7.32).
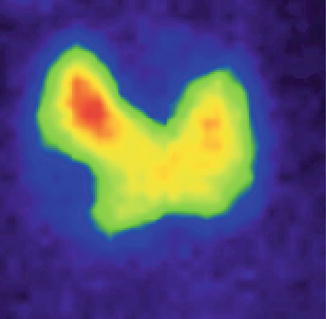

Fig. 7.32
Thyroid scintigraphy with Tc-99m Pt, AP incidence, which reveals a simple cyst of the right thyroid lobe, with no radiotracer uptake
Conclusion:
Right “cold” thyroid nodule. FNAB and cyst evacuation recommended.
The place of the thyroid Tc-99m Pt scan in the protocol of evaluation of thyroid nodules is nowadays very well established, being clearly reserved to specific clinical situations. The very large availability of noninvasive, highly sensitive thyroid US and the performance of the FNAB, of cytogenetic studies, lead to a more conservative approach with respect to the use of routine scintigraphy. The main indications are summarized in Fig. 7.33.
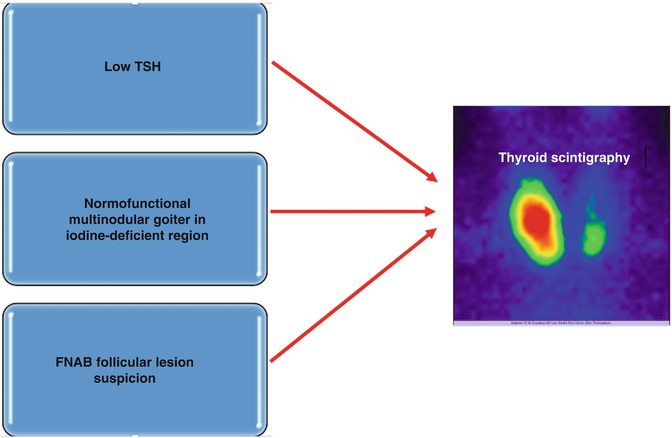

Fig. 7.33
Main indications of thyroid scintigraphy in the evaluation of thyroid nodules
Key Points
The cystic structure of a thyroid nodule is easily confirmed at thyroid ultrasound, a fact that does not recommend the necessity of thyroid scintigraphy furthermore.
In the case of a thyroid cyst, the FNAB has the role of diagnosis and also of therapy, with the evacuation of the cyst being performed in the same medical act.
The presence of some hypoechogenic patterns in the cystic structure leads to other associated processes: hemorrhage in the cyst, organization of hematoma with fibrosis, or the appearance of hemorrhage in a thyroid tumor nodule. In this situation, the scintigraphy may help to orient the physician to perform the FNAB. The nuclear test is not the first-line indication; in fact FNAB will set the differential diagnosis.
The indication of scintigraphy at a breastfeeding female is limited.
In the particular case described above, the indication of scintigraphy was related to the initial FNAB, which was negative (normal hematic cells and colloid). The increasing size and the appearance of lymph nodes suggested that it was not a simple cyst. The image obtained at nuclear test showed the intra-cystic tissue. The next sequence was the lymph node, and the FNAB showed metastatic papillary malignant cells.
The procedures related to breastfeeding were as follows: before the examination, the milk was taken off and kept for use after the test; after the tracer injection, the milk was expressed for the next 12 h but not used for the child nourishment. The breastfeeding continues normally after that interval.
7.3.5.2 Thyroid Scintigraphy with Tc-99m DMSA-Tc-99m (V) DMSA Gamma Camera
Radiopharmaceutical:
Technetium-99m pentavalent dimercaptosuccinic acid
Principle:
Tc-99m Pt is trapped from the bloodstream and fixed by the tissues with high metabolic activity, for example, the malignant cells.
Technique:
Patient preparation: No special preparation needed.
No need for fasting
No need for thyroid hormone withdrawal
No influence caused by other drugs
No influence caused by recent procedures with iodine contrast substances
Attention to breastfeeding and childbearing-age patients
Dose: 5–10 mCi (185–370 MBq)/patient of Tc-99m (V) DMSA.
Injected I.V.
1 h is necessary for waiting, after the injection for the first image.
Patient position: Supine.
Gamma camera.
Low-energy general-purpose (LEGP) collimator
Acquisition:
Whole-body planar acquisition.
First image after 1 h and the next after 4 h.
Anterior and posterior position.
1024 × 256 matrix.
Min 500,000 counts/image.
Also, spot images on the region of interest may be obtained.
Processing: There are special PC programs for image processing; additional may be used for the analysis of counts in different regions of interest (ROI); image interpretation
Clinical applications:
Diagnosis and follow-up of medullary thyroid carcinoma
Follow-up in non-avid iodine differentiated thyroid carcinoma
Necessary additional examinations:
Thyroid ultrasound
Serologic tests: Calcitonin in medullary thyroid carcinoma and thyroglobulin and antithyroglobulin in differentiated thyroid cancer
Fine needle aspiration biopsy (FNAB)
CT and MRI
PET/CT with specific neuroendocrine tracer
Comments:
It is a complementary method in the evaluation of persistent disease, which failed to be localized by other classic techniques.
Reports:
The reports respect the general format of the department with all the identification data of the patient, the institution, and the physician.
The report will include the technical data related to the radiopharmaceutical, the used dose, the type of gamma camera, and the acquisition data.
The report will include a description of the presence or absence of pathologic uptakes.
7.3.5.3 Thyroid Scintigraphy with Tc-99m MIBI Gamma Camera
Radiopharmaceutical:
Technetium-99m MIBI
Other names: Tc-99m-Sestamibi, Tc-99m-Hexamibi, Tc-99m 2 Methoxy 2 methylpropylisonitrile, Tc-99m Methoxy 2 isobutyl isonitrile, Tc-99m Sestamibi Chloride, and Cardiolite
Principle:
Tc-99m MIBI is a well-known lipophilic monovalent cation that shows an increased uptake in epithelial cells containing high numbers of mitochondria. For this reason, increased retention of MIBI is currently employed to detect hyperfunctioning parathyroid tissue and may be observed in benign or malignant thyroid tumors, especially of oncocytic nature.
Increased MIBI retention has also been described in hyperfunctioning thyroid tissue (toxic adenoma or toxic diffuse goiter), and this phenomenon is believed to be the consequence of increased mitochondria number in hypermetabolic cells. On the other hand, MIBI accumulation is reduced, or absent, in apoptotic or necrotic processes involving mitochondrial membrane potential collapse.
Technique:
Patient preparation: No special preparation needed.
No need for fasting
No need for thyroid hormone withdrawal
No influence caused by other drugs
No influence caused by recent procedures with iodine contrast substances
Attention to breastfeeding and childbearing-age patients (see chapter of Radioprotection)
Dose: 5–10 mCi (185–370 MBq)/patient of Tc-99m MIBI.
Injected I.V.
Early images at 1, 2, 5, and 15 min and delay at 1 h.
Patient position: Supine.
Gamma camera.
Low-energy general-purpose (LEGP) collimator
Acquisition:
Thyroid spot view or whole-body planar acquisition.
Anterior and posterior images.
Spot images on the region of interest may also be obtained.
1024 × 256 matrix in WBS or 128 × 128 in spot views.
Min 200,000 counts/image.
Processing: There are special PC programs for image processing; additional analysis of counts in different regions of interest (ROI) may be used.
Clinical applications:
Diagnosis and follow-up of medullary thyroid carcinoma
Follow-up in non-avid iodine differentiated thyroid carcinoma
Differential diagnosis between amiodarone-induced thyrotoxicosis type I (AIT I), amiodarone-induced thyrotoxicosis type II (AIT II), and amiodarone-induced thyrotoxicosis type III (AIT III).
Differential diagnosis of indeterminate FNAB results of thyroid nodules
Necessary additional examinations:
Thyroid ultrasound
Serologic tests: Calcitonin in medullary thyroid carcinoma and thyroglobulin and antithyroglobulin in differentiated thyroid cancer, TSH, FT4, and TRab
Fine needle aspiration biopsy (FNAB)
CT and MRI
PET/CT with specific neuroendocrine tracer
Comments:
It is a complementary method in the assessment of AIT I, AIT II, and AIT III and treatment decision.
Due to different patterns of action of amiodarone in AIT, Tc-99m MIBI scans will show a normal/intense uptake of the tracer in AIT I and a decreased/lack of activity in AIT II. There is also a very rare AIT type III, mixt, where the MIBI scan is also essential: early images show low/no uptake and delayed images also low or a very faint uptake. Even if AIT is considered a rare complication of the frequently used antiarrhythmic drug, the disease is very difficult to be managed in many situations. The MIBI scan is a noninvasive, simple, and easy method to differentiated the AIT types, in order to establish the best therapy for these patients.
The thyroid scan with Tc-99m MIBI may be used in differential diagnosis of benign/malignant nodules, especially with doubtful cytology. The malignant suspect nodules will present the same uptake in early and delayed images, while the benign ones will decrease their activity in the delayed images (Figs. 7.34 and 7.35).
Recent articles present the usefulness of the method in the differential diagnosis of benign/malignant thyroid nodules, especially those with indeterminate cytology of FNAB.
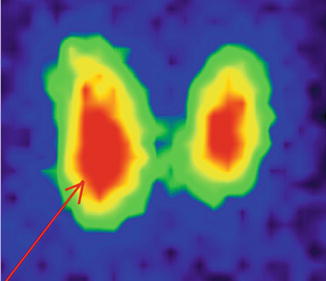
Fig. 7.34
Early image (15 min): nodule with intense uptake—highly probable benign
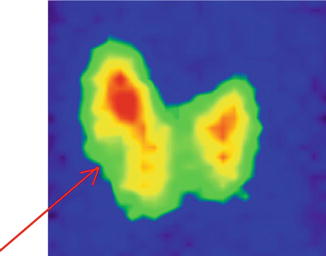
Fig. 7.35
Delayed image at 3 h: nodule with decreased uptake—highly probable benign
Giovanella et al. (2016) proposed a specific method for static thyroid scintigraphy where images were acquired 10 and 60 min after intravenous injection of 200 MBq of Tc-99m MIBI and visually assessed. Additionally, the MIBI washout index was calculated using a semiquantitative method. The team published that Tc-99m MIBI scintigraphy was superior to mutation analysis performed on patients with indeterminate cytology on FNAB, subsequently operated on to confirm or exclude a malignant lesion. Mutations for KRAS, HRAS, NRAS, and BRAF and translocations of PAX8/PPARγ, RET/PTC1, and RET/PTC3 were investigated.
The authors proposed a visual and a semiquantitative assessment of the nodules, in order to compare the risk of malignancy for the follicular neoplasm obtained at FNAB. The semiquantitative method consists of the following workflow: on each early image of MIBI thyroid scan, a region of interest (ROI) was drawn over the perimeter of the thyroid nodule and then moved to the opposite normal lobe; additionally, background ROIs were drawn above the inferior pole of thyroid lobe. These ROIs were then copied from the early to the delayed images. The mean count in each ROI was determined, and the early and delayed uptake results (EUR and DUR) were calculated by subtracting the count in the normal tissue (after correction for background activity) from the nodule count. The washout index (WOInd) was then calculated using the formula: WOInd = [(DUR/EUR × 100) − 100.
The visual assessment compares the Tc-99m pertechnetate and Tc-99m MIBI (early and delayed) thyroid images of a given patient. The authors recommended the following scoring system: pattern 1 no increased uptake on Tc-99m MIBI within the nodule in comparison to the Tc-99m pertechnetate scan on either the early or delayed image, pattern 2 increased uptake on the early image that had decreased on the delayed image, and pattern 3 increased uptake on the early image that remained unchanged or had further increased on the delayed image. Giovanella et al. demonstrated that pattern 3 was considered suspicious for malignancy and patterns 1 and 2 were both considered negative for malignancy of the thyroid nodule concerned.
Reports:
The reports respect the general format of the department with all the identification data of the patient, the institution, and the physician; the technical data related to the radiopharmaceutical, the used dose, the type of gamma camera; and the acquisition data; the report will note the estimation of the absorbed dose due to this examination. These data are available in predefined forms calculated for the majority of tracers and investigations. The report will include a description of the presence or absence of pathologic uptakes.
Normal image:
Same features as other thyroid Tc-99m scans
Case 1: Severe Amiodarone-Induced Hyperthyroidism
History:
A 61-year-old male, without previous history of neck irradiation; no cases of familial thyroid cancer, with a history of 14 years of severe permanent atrial fibrillation submitted to radiofrequency ablation. After the first ablation in 2015, the patient has administered repeatedly large doses of amiodarone, between December 2015 and February 2016, a fact that leads to a very aggressive form of amiodarone-induced hyperthyroidism. It was decided to stop the intake of amiodarone, mainly because of the lack of cardiac response.
The patient developed an amiodarone-induced thyrotoxicosis type III (mixt), a very rare and confusing clinical situation, in a previously normal thyroid gland, with no nodules. The treatment consisted both of synthetic antithyroid drugs (carbimazole 65 mg/day) and large dose of prednisone (65–80 mg/day). The thyroiditis was with severe clinical expression and with massive thyroid hormones storm, very difficult to be controlled.
Clinical examination:
Symptoms: insomnia, sweating, and anxiety. No other clinical symptoms with relation to the thyroid.
The neck examination did not reveal any tumor mass in the anterior neck area; thyroid with elastic structure, ascending while patient has deglutition; no clinical evidence of lymph nodes. No signs of exophthalmia. The heart rate was 110–140/min.
Examinations:
The ultrasound revealed a diffuse destructive hypoechogenic thyroid structure, highly vascularized, with a microcyst of 3 mm in the left thyroid lobe. No pathologic lymph nodes (Fig. 7.36).
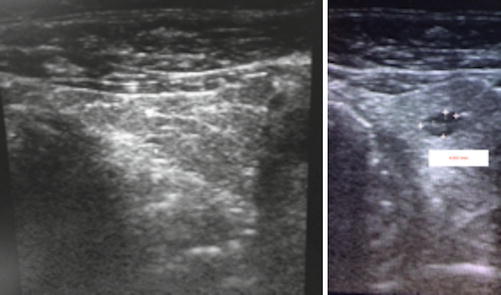

Fig. 7.36
Thyroid ultrasound gray-scale showing the important thyroid tissue destruction and microcyst in the left lobe; patient with AIT
TSH < 0.001 mIU/L (N.V. 0.4–4.5 mIU/L), very low and undetectable.
Anti-TPO > 600 mIU/L (N.V. <34 mIU/L), very increased.
Anti-Tg—1280 kIU/L (N.V. <115 kIU/L), very increased.
FT4 > 100 pmol/L (N.V. 12–22 pmol/L),very increased.
EBR—12 mm/h, normal.
TRab negative.
Interleukin-6—6.3 (N.V. 5–15 pmol/L), normal; the value was against the type II AIT.
Iodemia—1762 (N.V. 46–71 μg/L), very increased.
Urinary iodine—10,464 (N.V 200–299 μg/L), very increased.
Findings:
The scintigraphy with Tc-99m Pt showed a very low uptake, without any thyroid tissue active in the neck area. There was very low Tc-99m Pt uptake (0.1%) while the normal values are 0.7–1.4% (Fig. 7.37). The scintigraphy with Tc-99m MIBI performed next day depicted the diagnosis between the types of AIT I, II, and III. Figure 7.38a shows the thyroid scan with very low uptake on early image and also low uptake in delayed images (Fig. 7.38b), suggestive for AIT type II/III, despite the lack of expression of serologic inflammatory factors. The results were crucial for the adequate selection of therapy.
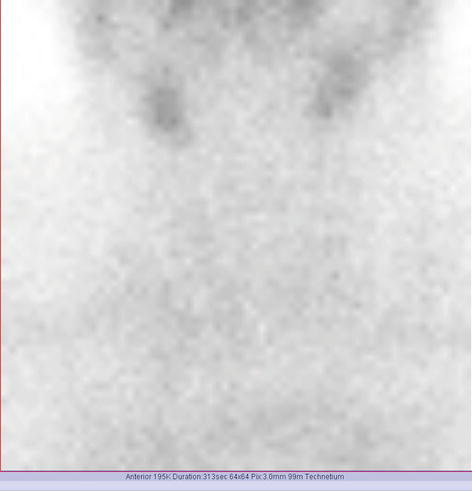
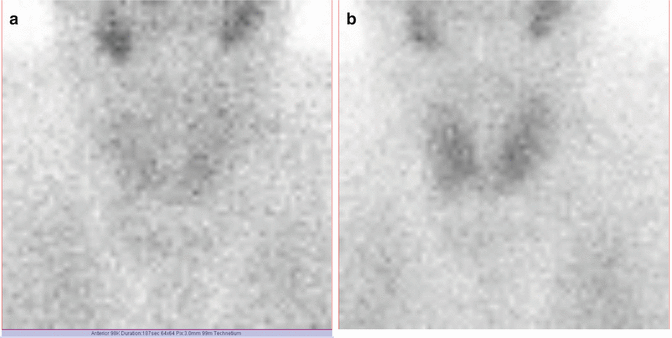

Fig. 7.37
Thyroid Tc-99m Pt scan showing the low uptake in the cervical area due to important thyroid tissue destruction; patient with AIT

Fig. 7.38
Thyroid Tc-99m MIBI scan early image (a) and delayed image (b) showing the low uptake on both sequences, in the cervical area due to important thyroid tissue destruction; patient with AIT type II
Conclusion:
Amiodarone-induced hyperthyroidism. The Tc-99m MIBI scan is essential to differentiate AIT type I which needs antithyroid drugs from the AIT type II or III which needs corticotherapy.
7.3.5.4 Thyroid Uptake with Tc-99m Pertechnetate (Tc-99m Pt) Gamma Camera
Radiopharmaceutical:
Technetium-99m pertechnetate (Tc-99m Pt)
Principle:
Thyroid uptake determination is the measurement of the fraction of an administered amount of radioactive tracer that accumulates in the thyroid at selected times, after the intravenous administration of Tc-99m pertechnetate. Thyroid uptake calculates the area, volume, weight, and split uptake of the thyroid.
Technique:
Patient preparation: No special preparation needed.
No need for fasting
No need for thyroid hormone withdrawal
No influence caused by other drugs
No influence caused by recent procedures with iodine contrast substances
Attention to breastfeeding patients, children, and potential fetal exposure (see chapter Radioprotection)
Dose: 2–10 mCi (74–370) MBq/patient of Tc-99m Pt obtained from the generator of Tc-99m in the department, respecting the quality control requirements.
Dose calibration.
Injected I.V.
15–30 min are necessary for waiting, after the injection for the scan.
Patient position: Supine, slight neck extension.
Gamma camera:
Calibration and quality valid control tests
Small-field rectangular (optional pinhole collimator)
Low-energy high-resolution (LEHR) collimator
Anterior-posterior (AP) over the patient’s neck, as close to the neck as possible, avoiding the influence of resolution by an inadequate distance
There are two methods of acquisition:
Syringe images method—uses counts obtained from syringe images.
Fig. 7.39
Preinjection registered counts
Fig. 7.40
Postinjection registered counts
Fig. 7.41
Thyroid scan Tc-99m Pt uptake workflow
Dose calibrator and syringe images method
Measures the activity of the full syringe.
Measures the activity of the empty syringe following patient injection.
Time and date when the full and empty syringe was measured.
When using the dose calibrator method, a new calibration factor is needed each time.
The camera parameters are:
Anterior position using gamma camera
Selected energy 140 keV
Window 15–20%
128 × 128 matrix or 256 × 256
100,000–250,000 counts/image or 5 min
Processing: There are special PC programs; the uptake values are obtained in workflow factory templates, present in the soft of the cameras.
Clinical applications:
Assists in determining the amount of I-131 to be administered to patients for therapy of hyperthyroidism due to Graves’ disease or toxic nodular goiter. The uptake should be performed as close as possible in time to the treatment.
Differentiates subacute or painless thyroiditis and factitious hyperthyroidism from Graves’ disease and other forms of hyperthyroidism.
Assists in diagnosing and confirming the diagnosis of amiodarone-induced hyperthyroidism.
Necessary additional examinations:
Clinical examination; remember to discuss and to examine the patient before the injection, in order to limit the hazardous radiation exposure.
Serologic tests: TSH, FT4, FT3, antibodies TPO, TRab, etc.
Comments:
The uptake also justifies the performance of the scan. In addition, with the same dose, we are able to obtain the structural information.
Reports:
The reports respect the general format of the department with all the identification data of the patient, the institution, and the physician.
The report will include the technical data related to the radiopharmaceutical, the used dose, the type of gamma camera, and the acquisition data. The report will note the estimation of the absorbed dose due to this examination. These data are available in predefined forms calculated for the majority of tracers and investigations. The report will include the value of the uptake and also the reference range of values, largely variable in different zones, areas, and countries (Fig. 7.42).
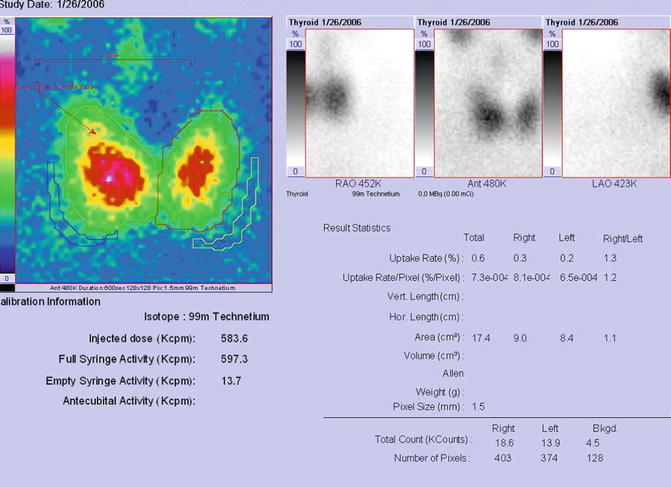
Fig. 7.42
Report of Tc-99m Pt thyroid uptake
Most frequently, the normal thyroid uptake values determined by Tc-99m Pt uptake are reported to be between 0.5 and 1.6%.
Remember to ask from the nuclear medicine department the normal range established by the department standard. These values are set up according to some specific conditions: race, gender, age, geographical area, iodine-deficient region, etc.
7.3.5.5 Thyroid Uptake with I-131 or I-123 Sodium Iodide
Radiopharmaceutical:
Radioiodine I-131 or I-123 sodium iodide capsules or liquid
Principle:
Thyroid uptake determination is the measurement of the fraction of an administered amount of radioactive tracer that accumulates in the thyroid at selected times, after orally administered radioiodine. Radioiodine thyroid uptake (RAIU) calculates the uptake of the thyroid.
Technique:
Patient preparation: Special preparation needed
It is necessary to have at least 4–6 h for fasting.
It is necessary to have a thyroid hormone withdrawal: for antithyroid drugs, 3–7 days, and for substitutive thyroid hormones, 14–20 days.
There can be an influence caused by other drugs.
Avoid or limit procedures with iodine contrast substances.
Low-iodine diet may be recommended at least 3–7 days before the test.
Attention to breastfeeding patients, children, and potential fetal exposure (see the chapter Radioprotection).
The amount of radioiodine is similar to daily intake of iodine, so the allergic side effects are very unusual and improbable.
Dosage:
50–100 μCi (1.85–3.7 MBq)/patient of I-131 NaI
100–400 μCi (3.7–14.8 MBq)/patient of I-123 NaI
Dose calibration.
Orally administrated capsules or liquid.
Measurements may be started at 2–5–6–24 h.
Patient position: Supine, slight neck extension.
Uptake system:
Calibration and valid quality control tests.
Immediate dose measurement, prior to patient administration, in a phantom model mimics the neck and the thyroid; the precise time and number of counts emitted by the dose are registered.
The capsules are swallowed with a little amount of water.
The measurement of counts emitted by the thyroid neck starts at 2 h for I-131 and at 6 h for I-123, and they are repeated in the same conditions after 24 h. There may be also other standard supplementary intervals created by the department.
Processing: There are special PC programs; the uptake values are obtained in workflow factory templates present in the soft of uptake system or simply by calculating the ratio:
The details of the ratio are related to the measurement of the background and of the decay.
The formula is:

The decay correction factor is 0.918 for I-131 at 24 h, 0.730 for I-123 at 6 h, and 0.284 at 24 h (see Chap. 6).
Clinical applications:
Assists in determining the amount of I-131 to be administered to patients for the therapy of hyperthyroidism due to Graves’ disease or toxic nodular goiter. The uptake should be performed as close in time as possible to the treatment.
Differentiates subacute or painless thyroiditis and factitious hyperthyroidism from Graves’ disease and other forms of hyperthyroidism.
Assists in diagnosing and confirming the diagnosis of amiodarone-induced hyperthyroidism.
Tests of suppression.
The measurement of the uptake is of limited value in the case of a hypothyroidism diagnosis.
Necessary additional examinations:
Clinical examination; remember to discuss and to examine the patient before the administration of the capsule, in order to limit the hazardous radiation exposure.
Serologic tests: TSH, FT4, FT3, antibodies TPO, TRab, etc.
Comments:
The uptake also justifies the performance of the scan. In addition, the dose permits to obtain the structural information.
Reports:
The reports respect the general format of the department with all the identification data of the patient, the institution, and the physician. The report will include the technical data related to the radiopharmaceutical, the used dose, the type of uptake system, and the acquisition data. The report will note the estimation of the absorbed dose due to this examination. These data are available in predefined forms calculated for the majority of tracers and investigations.
The report will include the value of the uptake and also the reference range of values, largely variable in different zones, areas, and countries.
Normal values:
At 2–6 h, the range is 3–8–15%.
At 24 h, the range is between 5 and 35% in most regions of Northern America, and in many other parts of the world, the range is 15–50%.
Some laboratories only measure RAIU at 24 h, which is the most accurate in many circumstances.
Remember to ask the laboratory for the normal values set up for that specific region.
Increased RAIU:
Hyperthyroidism (Graves’ disease, Plummer’s disease-toxic adenoma, trophoblastic disease, pituitary resistance to thyroid hormone, TSH-producing pituitary adenoma)
Nontoxic goiter (endemic, inherited biosynthetic defects, generalized resistance to thyroid hormone, some cases of Hashimoto’s thyroiditis)
Excessive hormonal loss (nephrosis, chronic diarrhea, hypolipidemic resins, diet high in soybean)
Decreased renal clearance of iodine (renal insufficiency, severe heart failure)
Recovery of the suppressed thyroid (withdrawal of thyroid hormone and antithyroid drug administration, subacute thyroiditis, iodine-induced myxedema)
Iodine deficiency (endemic or sporadic dietary deficiency, excessive iodine loss as in pregnancy or in the dehalogenase defect)
TSH administration
Decreased RAIU:
Hypothyroidism (primary or secondary); Hashimoto’s thyroiditis
Defect in iodide concentration (inherited “trapping” defect, early phase of subacute thyroiditis, transient hyperthyroidism)
Suppressed thyroid gland caused by thyroid hormone (hormone replacement, thyrotoxicosis factitia, struma ovarii)
Iodine excess (dietary, drugs, and other iodine contaminants)
Miscellaneous drugs and chemicals (see Table 7.2)
Table 7.2
Most frequent interfering drugs with the thyroid uptake
Interfering drug | Withhold | Study affected |
|---|---|---|
Antithyroid medications | 1 week | Decreases thyroid uptake |
Thyroid hormones (T3, T4, or T3+T4) | 2–3 weeks | Decreases thyroid uptake |
Expectorants, vitamins | 2 weeks | Decreases thyroid uptake |
Phenylbutazone | 1–2 weeks | Decreases thyroid uptake |
Salicylates | 1 week | Decreases thyroid uptake |
Steroids | 1 week | Decreases thyroid uptake |
Sodium nitroprusside | 1 week | Decreases thyroid uptake |
Antihistamines | 1 week | Decreases thyroid uptake |
Antiparasitics | 1 week | Decreases thyroid uptake |
Penicillin | 1 week | Decreases thyroid uptake |
Sulfonamides | 1 week | Decreases thyroid uptake |
Tolbutamide | 1 week | Decreases thyroid uptake |
Thiopental
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|