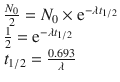(1)
Institute of Oncology “Prof. Ion Chiricuță”, Nuclear Medicine & Endocrine Tumors, Cluj-Napoca, Romania
“If anything is sacred, the human body is sacred”
Walt Whitman, (poet 1819–1892)
9.1 Overview
The two suprarenal glands, which are also called adrenal glands, are retroperitoneal organs that lie on the upper poles of the kidneys. They are surrounded by renal fascia but are separated from the kidneys by the perirenal fat. The combined weight of suprarenal glands in human adults ranges from 7 to 10 g. The right suprarenal gland is pyramid shaped and caps the upper pole of the right kidney. It lies behind the right lobe of the liver and extends medially behind the inferior vena cava. It rests posteriorly on the diaphragm. The left adrenal is crescent shaped and lies medial to the kidney above the left renal vein. It lies behind the pancreas, the lesser sac, and the stomach and rests posteriorly on the diaphragm.
A conjunctive capsule wraps the adrenal gland, composed of two distinct parts:
Cortex—the peripheral area
Medulla—the core area
The cortex represents 80–90% and is composed of cords of epithelial cells that are directly related to the blood vessels (capillary sinusoids). These epithelial cells are grouped so that different adrenal cortex shows a characteristic layering in three areas arranged as follows:
Zone glomerulosa—a thin layer of cells that lies just underneath the capsule, constitutes about 15% of the adrenal cortex. These cells are the only ones in the adrenal gland capable of secreting significant amounts of aldosterone because they contain the enzyme aldosterone synthase, which is necessary for the synthesis of aldosterone. The secretion of these cells is mainly controlled by the extracellular fluid concentrations of angiotensin II and potassium, both of which stimulate aldosterone secretion.
Zone fasciculata—the middle and widest layer, constitutes about 75% of the adrenal cortex and secretes the glucocorticoids cortisol and corticosterone, as well as small amounts of adrenal androgens and estrogens. The secretion of these cells is controlled in large part by the hypothalamic-pituitary axis via the adrenocorticotropic hormone (ACTH).
Zone reticularis—the deep layer of the cortex, secretes the adrenal androgens dehydroepiandrosterone (DHEA) and androstenedione, as well as small amounts of estrogens and some glucocorticoids. ACTH also regulates secretion of these cells, although other factors such as cortical androgen-stimulating hormone, released from the pituitary, may also be involved.
Some of the actions produced by these hormones include:
Cortisol hormone—also known as hydrocortisone, controls the body’s use of fats, proteins, and carbohydrates.
Corticosterone—this hormone, together with cortisol hormones, suppresses inflammatory reactions in the body and also affects the immune system.
Aldosterone hormone—this hormone inhibits the level of sodium excreted into the urine, maintaining blood volume and blood pressure.
Androgenic steroids (androgen hormones)—these hormones cause retention of Na (protein anabolic effect) and stimulate osteogenesis and somatic development.
The suprarenal medulla (MSR) is the core of the gland and represents 20% of it, and the cortex surrounds it.
MSR is composed of chromaffin cells, arranged in groups or in columns in which there are large capillary sinusoids and veins of different sizes. Among the chromaffin cells, scattered sympathetic ganglion cells are found, alone or in groups. These cells are neurons which have lost their axons and gained secretory properties and therefore can be considered as a medullosuprarenal sympathetic ganglion.
Medullosuprarenal hormones are called catecholamines, and they are represented by adrenalin (80%) and noradrenalin (20%):
Adrenalin (also called epinephrine)—This hormone increases the heart rate and force of heart contractions, facilitates blood flow to the muscles and brain, causes relaxation of smooth muscles, and helps with conversion of glycogen to glucose in the liver and other activities.
Noradrenalin (also called norepinephrine)—This hormone has little effect on the smooth muscle, metabolic processes, and cardiac output but has strong vasoconstrictive effects, thus increasing blood pressure.
9.2 Pathophysiology
9.2.1 Pathologies of the Adrenal Cortex
9.2.1.1 Hypoadrenalism (Adrenal Insufficiency): Addison’s Disease
This disease results from an inability of the adrenal cortices to produce sufficient adrenocortical hormones, and this in turn is most frequently caused by primary atrophy or injury of the adrenal cortices. In about 80% of the cases, the atrophy is caused by autoimmunity against the cortices. The adrenal gland hypofunction is also frequently caused by tuberculosis destruction of the adrenal glands or by cancer invasion of the adrenal cortices.
The symptoms of Addison’s disease develop insidiously. The most common symptoms are fatigue, lightheadedness upon standing or while upright, muscle weakness, fever, weight loss, difficulty in standing up, anxiety, nausea, vomiting, diarrhea, headache, sweating, changes in mood and personality, and joint and muscle pains. Some have marked cravings for salt or salty foods due to the urinary losses of sodium. Affected individuals may note increased tanning since adrenal insufficiency is manifested in the skin primarily by hyperpigmentation, specifically due to the decrease in cortisol and subsequent increase in ACTH levels.
9.2.1.2 Hyperadrenalism: Cushing’s Syndrome
Hypersecretion by the adrenal cortex causes a complex cascade of hormone effects called the Cushing’s syndrome. Many of the abnormalities of Cushing’s syndrome are ascribable to abnormal amounts of cortisol, but excess secretion of androgens may also cause important effects. Hypercortisolism can occur from multiple causes, including:
Adenomas of the anterior pituitary that secrete large amounts of ACTH, which then causes adrenal hyperplasia and excess cortisol secretion
Abnormal function of the hypothalamus that causes high levels of corticotropin-releasing hormone (CRH), which stimulates excess ACTH release
“Ectopic secretion” of ACTH by a tumor elsewhere in the body, such as an abdominal carcinoma
Adenomas of the adrenal cortex
When the Cushing’s syndrome is secondary to excess secretion of ACTH by the anterior pituitary, this is referred to as Cushing’s disease.
A special characteristic of Cushing’s syndrome is the mobilization of fat from the lower part of the body, with concomitant extra deposition of fat in the thoracic and upper abdominal regions, giving rise to a buffalo torso. The excess secretion of steroids also leads to an edematous appearance of the face, and the androgenic potency of some of the hormones sometimes causes acne and hirsutism (excess growth of facial hair). The appearance of the face is frequently described as a “moon face.” About 80% of patients have hypertension, presumably because of the mineralocorticoid effects of cortisol.
9.2.2 Pathologies of the Adrenal Medulla: The Pheochromocytoma
Pheochromocytoma is a neuroendocrine tumor of the medulla of the suprarenal glands originating in the chromaffin cells and secrets excessive amounts of catecholamines, usually noradrenalin, and adrenalin to a lesser extent. In general, they are benign tumors, but there are situations when they might be malignant.
The signs and symptoms of a pheochromocytoma are those of sympathetic nervous system hyperactivity, including:
Skin rash
Flank pain
Elevated heart rate; elevated blood pressure, including paroxysmal (sporadic, episodic) high blood pressure, which sometimes can be more difficult to detect
Orthostatic hypotension
Anxiety often resembling that of a panic attack
Excessive sweating
Headaches
Elevated blood glucose level (due primarily to catecholamine stimulation of lipolysis (breakdown of stored fat) leading to high levels of free fatty acids and the subsequent inhibition of glucose uptake by muscle cells; further, stimulation of beta-adrenergic receptors leads to glycogenolysis and gluconeogenesis and thus elevation of blood glucose levels)
The diagnosis can be established by measuring catecholamines and metanephrines in plasma (blood) or through a 24-h urine collection, and surgical resection of the tumor is the treatment of first choice, either by open laparotomy or else laparoscopy.
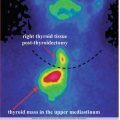
The evaluation of adrenal disorders has been simplified by the development of sensitive and specific biochemical tests and by the availability of high-resolution CT and MR imaging. On the other hand, the exquisite spatial resolution of these imaging modalities has produced the diagnostic conundrum of the adrenal incidentaloma. The scintigraphic assessment of disorders of the adrenal cortex, such as Cushing’s syndrome, primary aldosteronism, and adrenal hyperandrogenism, is only infrequently required, whereas the disease in patients with pheochromocytoma has resulted in an expanding clinical role for adrenal medullary imaging.
9.3 Adrenal Cortical Scintigraphy
I-131-6β-Iodomethyl-19-norcholesterol (also known as iodocholesterol or NP-59) is the current radiopharmaceutical of choice for adrenal cortical imaging. The main disadvantage of iodocholesterol scintigraphy is the high radiation dose, due to beta component of I-131. The uptake of these agents into adrenocortical cells is related to the precursor status of cholesterol for adrenal steroid synthesis and to the transportation of cholesterol and radiocholesterol by low-density lipoprotein (LDL). An increase in the serum cholesterol reduces the uptake by downregulating LDL receptors. Any increase in circulating ACTH results in increased radiocholesterol uptake. Although radiocholesterol is stored in adrenal cortical cells, it is not esterified and therefore not incorporated into adrenal hormones. Several medications, including glucocorticoids, diuretics, spironolactone, ketoconazole, and cholesterol-lowering agents, may interfere with radiocholesterol uptake. Following injection, the uptake of the radiocholesterols is progressive over several days, and there is prolonged retention within the adrenal cortex, permitting imaging over a period of days to weeks. Although adrenal uptake for all of these agents is ≤0.2% per gland, total body exposure is relatively high:
Radiopharmaceutical: I-131—iodocholesterol
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree