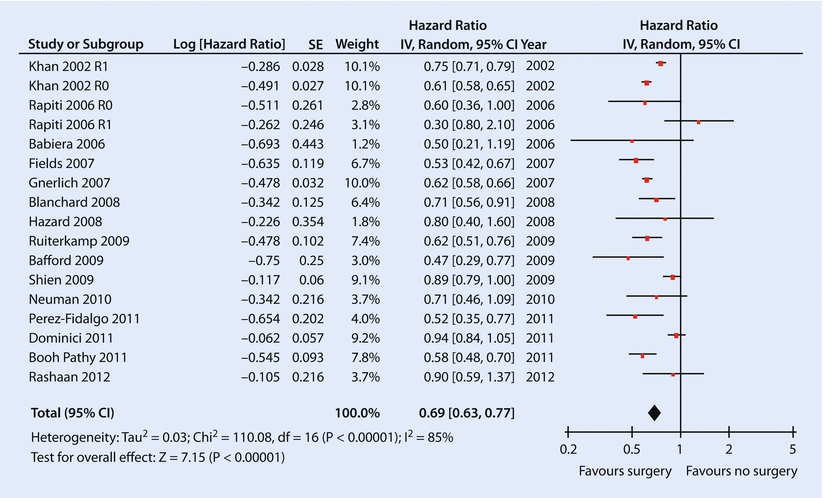
Fig. 55.1
Forest plot of meta-analysis by Petrelli and Barni, including 15 retrospective studies that examined the association between primary site local therapy and survival in de novo stage IV breast cancer patients (Reprinted from Petrelli and Barni [42], Fig. 2, page 3288 [42] with permission from Springer)
It is difficult to assess the benefit of postoperative versus primary radiotherapy (RT) as not all studies distinguish delivery and rate consistently. One of the larger studies by Le Scodan and colleagues identified patients with de novo stage IV breast cancer treated over a 20-year period. Of this cohort, 320 received locoregional RT, 30 received only surgery, and 41 women received both surgery and RT, which was delivered to both breast/chest wall and nodal fields with a boost to the tumour site for most patients. The overall survival rate was improved by a third in the group receiving PSLT versus those who did not, with an adjusted hazard ratio of 0.7 (95% CI 0.58–0.85) [31]. In another cohort of 733 patients evaluated in the British Columbia study from 1996 to 2005, 378 patients had PSLT which consisted of surgery alone in 67% of patients, radiotherapy alone in 22%, and both in 11%. The 5-year overall survival rates were 21% versus 14% (p < 0.001), and the rates of locoregional progression-free survival were 72% versus 46% (p < 0.001) [36]. These studies suggest that patients that had surgery had higher rates of RT delivery as well as some benefit in OS and progression-free survival.
Very limited information is available regarding chest wall outcomes. However, the largest study by Hazard and colleagues [34] evaluated 111 patients presenting with stage IV breast cancer. Early compared to delayed or no surgical resection of the primary tumour reduced symptomatic chest wall disease by 86%, but there was no statistically significant difference in terms of overall survival. It was also noted that patients who maintained a controlled primary (whether through local or systemic therapy) had better survival outcomes (HR = 0.42, P < 0.002) compared to those with uncontrolled primary sites.
55.3.2 Potential Biases
The results of the retrospective studies have to be reviewed closely as they carry some significant bias that lead to the concern that the demonstrated survival from local therapy may be due to patient selection. The use of surgery was higher in women who have more favourable profile such as young age, smaller tumours, non-visceral disease, fewer comorbidities [27, 39, 40], and better access to care [25, 33]. Additionally, it is likely that the timing of surgery may introduce bias [27, 28, 32, 44]. For example, if women with T1–2 tumours, for whom a preoperative metastatic survey is not standard of care, were staged post-operatively because of a high nodal burden and were then discovered to have asymptomatic or low-volume metastatic disease, their prognosis may be better than women with symptomatic and high burden metastases diagnosed preoperatively. This consideration particularly affects tumour registry data, where the final stage is usually recorded several months after completion of primary therapy, by which time asymptomatic metastases may have been discovered. In addition, in any cohort study subtle differences in disease burden and patient fitness for intervention and disease biology may bias selection for surgery and hence outcomes.
55.4 Randomized Clinical Trials
Several randomized trials have been initiated in an effort to validate the results of the retrospective studies, particularly due to the concern that the apparent benefit of PSLT is driven by patient selection. A total of seven trials were initiated; two have completed accrual. Final results have been published by Badwe and colleagues from India, and preliminary results have been reported by Soran and colleagues from Turkey. Trials are ongoing in the USA/Canada (ECOG-ACRIN 2108) and Japan, whereas the Austrian trial (POSYTIVE, ABCSG 28) closed after accrual of 93 patients. Two additional trials were launched; the trial in the Netherlands closed with minimal accrual, and one in Thailand was withdrawn prior to accrual. Three of these trials (India, Japan, the USA/Canada) required induction systemic therapy prior to surgery.
55.4.1 Completed Trials
The first trial to be published was from India. It opened in 2005 at the Tata Memorial Cancer Institute in Mumbai (NCT00193778) [45]. Participants were enrolled from a pool of 716 patients, of whom only 25 had resectable primary tumours; of these, 440 responded to initial systemic therapy which consisted almost exclusively of chemotherapy (six cycles of anthracycline-based treatment in 96% with taxanes added in under 5% of patients). After exclusion of 90 subjects for a variety of reasons, 350 patients were randomized to PSLT versus continuation of systemic therapy. PSLT consisted of surgery with or without radiotherapy (when indicated). Primary outcomes were overall survival and disease-free survival. Secondary outcomes were locoregional progression-free survival (PFS), distant PFS, and health-related quality of life.
Of the 350 randomly assigned patients, 173 patients were assigned to PSLT, and 177 were assigned to continuation of systemic therapy without PSLT. The groups were well matched in terms of disease characteristics and demographics. Median duration of follow-up was 23 months with a total of 235 deaths at the data cut-off date. Patients who had locoregional or distant progression as the first event were censored at that time for locoregional PFS or distant PFS analysis, resulting in a shorter distant PFS in the PSLT arm. The final trial results show no difference in median overall survival (HR 1.04, 95% CI 0.80–1.34, ◘ Fig. 55.2); a series of pre-planned subset analyses also showed no significant differences in overall survival. Locoregional PFS was significantly better in the surgical group (HR 0.16, p < 0.001), whereas distant PFS was decreased in the PSLT arm compared to the continued systemic therapy arm, with a HR of 1.42. Overall, the trial showed no survival gain with surgical therapy for de novo stage IV breast cancer [45].
Of note, the median survival of the Tata Memorial trial population was 19 months, which is significantly lower than that observed in reports from developed countries, where recent stage IV trials show median overall survival to be in the range of 40 months (for hormone receptor-positive breast cancer) [46], and 49 months (in a prospective observational study of de novo stage IV breast cancer patients) [47]. Fewer than 30% of patients in the Tata Memorial trial had bone-only disease, and about one-quarter had three or fewer metastatic lesions. Following completion of protocol-directed locoregional therapy, the patterns of continued systemic therapy did not include HER2-directed agents for the most part (trastuzumab was used in 8/90 women with HER2-positive tumours). A shorter distant PFS was observed in the PSLT group, but this did not translate into a survival disadvantage.
In contrast to the Tata Memorial trial, the Turkish Federation of Breast Diseases study (NCT00557986) [48] was designed without an induction systemic therapy phase. A total of 274 patients were accrued and were randomized to surgery (mastectomy or lumpectomy followed by radiation therapy, with or without axillary dissection in patients with positive nodes) prior to systemic therapy or systemic therapy alone. The trial was powered to detect an improvement in 3-year survival of 18%. The primary outcome was overall survival, with secondary outcomes related to progression-free survival, quality of life measures, and morbidity related to locoregional therapy. According to results reported at the 2016 American Society of Clinical Oncology (ASCO) meeting, there was no difference in survival at 36 months (p = 0.5). However local therapy did improve survival at 40 months of median follow-up with a 9-month difference (46 vs 37 months). The overall 5-year survival rate was 42% among women who received locoregional therapy, compared to 25% in women who did not (HR 0.66; 95% CI 0.49–0.88; P = .005) The systemic therapy group had worse locoregional progression rate compared to the locoregional therapy group (11% vs 1%, p = 0.001). A number of unplanned subset analyses were conducted, which also showed a significant survival benefit in hormone receptor-positive disease, HER2-negative tumours, younger patients, and those with solitary bony lesions. Particularly for bone-only metastasis, the median survival was 56 months in the locoregional group versus 42 months in the systemic therapy-only group (HR 0.67; 95%CI 0.43–1.07; P = 0.09) with the important caveat that there was no tissue diagnosis of solitary bone lesions. Worse outcomes were noted for patients with TNBC (median overall survival of 16 vs 24 months in patient who did not have surgery) and those with multiple visceral metastases if surgery was performed, presumable due to the need to discontinue or delay systemic therapy to permit safe surgery [48].
55.4.2 Ongoing Trials
The US and Canada trial, run by the Eastern Cooperative Oncology Group (EA2108, NCT01242800) [49], closed in July 2015 with 383 patients accrued. Patients received induction systemic therapy consisting of endocrine, cytotoxic, or biologic regimens appropriate to the patient’s age and tumour type. Those who responded, or whose disease remained stable following 16–32 weeks of therapy with the induction regimen, were randomized to surgery and radiotherapy (as dictated by standards of care for non-metastatic patients) or to continuation of systemic therapy. The trial was powered to detect an overall survival difference of 19% at 3 years. The primary outcome is overall survival; secondary outcomes are local progression-free survival and quality of life. Biological samples are being banked for correlative studies. Importantly, this trial addresses the role of axillary clearance and radiation therapy.
A similar trial designed by the Japanese Clinical Oncology Group (JCOG 1017) [50] opened in June 2011; this too requires induction chemotherapy prior to locoregional therapy. After 3 months of systemic therapy, women who show no disease progression are randomized to undergo surgery or to continue systemic therapy; radiotherapy is not required. The primary outcome is overall survival. Secondary outcomes are local recurrence rate and local control rate. The trial has accrued 350 patients thus far with a target of 440.
The POSYTIVE trial in Austria (NCT01015625) [51] initially allowed randomization prior to initial locoregional therapy; however the protocol was subsequently revised to allow for systemic therapy prior to randomization. The accrual goal was 254 patients, but the study has closed with accrual of 93 patients due to slow accrual (personal communication, Prof. Florian Fitzal). Patients with synchronous metastatic breast cancer were randomly assigned to receive lumpectomy or mastectomy + axillary surgery /± radiotherapy versus not. Patients in the no-surgery group could have delayed surgery if necessary. Primary outcome is median overall survival. Secondary outcomes are time to distant progression and time to local progression.
55.5 Prospective Registry Trial
The Translational Breast Cancer Research Group has completed a prospective multi-institutional data registry gathering data on the modern management of stage IV breast cancer (TBCRC 0313) [52]. The goal of the registry is to record information on the incidence of uncontrolled local disease, metastatic disease, rate of surgical intervention for palliation for the intact primary (in patients not undergoing PSLT), the frequency and effect of uncontrolled chest wall disease, and quality of life. Biologic and molecular data has been collected and will be used to determine interactions between the primary and metastatic sites. A total of 127 eligible patients have been enrolled thus far and are categorized into cohorts of those with an intact primary (A) or those with a resected primary and metastases discovered within 3 months of surgery (B). All patients received first-line systemic treatment according to standard institutional guidelines. Preliminary results presented at ASCO in 2016 showed that for patients in cohort A, after a 54-month median follow-up, 3-year OS is 70% and is better for patients who are responders to chemotherapy compared to nonresponders (78% vs 24%, p = 0.001). However, amongst responders no benefit was noted with surgery in 3-year overall survival (p = 0.85) irrespective of tumour subtype, although HER2-positive status and response seem to provide longer survival (93%). In addition, surgery did not improve progression-free survival with the median time to progression at 13 versus 12 months [52]. The data also demonstrated that surgical palliation of the primary tumour is uncommon in the modern eras as the majority of patients are responders to first-line therapy. The results of this registry cohort study show trends in survival in metastatic breast cancer; however the role of surgery in de novo stage IV breast cancer is still an issue for debate. In a preliminary analysis of survival relative to the 21-gene Recurrence Score (RS), King and colleagues observed that a high RS was independently prognostic for 2-year OS (hazard ratio, 1.83; 95% CI, 1.14–2.95; P = 0.013), and this group of women had a shorter 2-year survival if treated with initial endocrine therapy rather than initial chemotherapy [47].
55.6 Conclusion
The mainstay of treatment for the patient with metastatic breast cancer is systemic therapy. The optimal role for primary site local therapy in individuals with an intact primary tumour remains to be determined. After a flurry of enthusiasm following multiple retrospective analyses showing an apparent benefit of PSLT, the first mature randomized trial addressing this question yielded null results in terms of overall survival although there appears to be a benefit in terms of local control [45]; while the second complete trial remains unpublished at this time, results presented at ASCO 2016 appear to show a survival benefit for the use of locoregional therapy for the primary tumour [48]. The results from other randomized trials are pending, and until these are available, practitioners should be circumspect in offering PSLT to patients with metastatic disease. The role of radiotherapy in conjunction with surgery or as primary therapy also remains to be determined. Locoregional management of the primary tumour in stage IV breast cancer patients remains appropriate in the presence of symptoms; it may also be appropriate in highly selected patients if the distant disease is well controlled and the primary site shows unequivocal evidence of progression. If the primary site is also responding to systemic therapy however, prospective data from the TBCRC and the Tata Memorial trial showed that very few patients require palliative surgery. If both local and distant sites are well controlled, the rationale for LRT is also weak, since the primary site is likely to remain well controlled for the patient’s life span. The only possible exception may be the patient who has had a complete response to systemic therapy at distant sites and would be rendered stage IV with no evaluable disease by resection of the primary tumour. In this setting, highly selected series show that a third or more patients may achieve long-term survival [50]. If PSLT is decided upon following the above considerations, breast conservation (if feasible) is clearly the least morbid approach, and the lack of expectation of a survival benefit should be clear to the patient. Finally, in making recommendations for primary site locoregional therapy, it is of utmost importance to consider the potential morbidity and the costs of therapy. Notably, guidelines from the European Society for Medical Oncology published in 2014 state that «that surgery of the primary (tumour) should not be offered as a routine practice but can be discussed on a case-by-case basis and offered to selected patients ». The basis for selection is not specified [53].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree