General
Patient’s inability to access a radiotherapy centre
Neurological/orthopaedic conditions (upper limb functional limitations, inability to keep still, etc.)
Severe lung disease
Severe cardiac disease for left-sided breast tumours
Germline TP53 mutations (Li-Fraumeni syndrome)
Specific AbsoluteRelative
Pregnancy
Patient’s inability to maintain stable treatment position
Active connective tissue diseases (systemic lupus erythematosus, dermatomyositis and scleroderma)
Quiescent connective tissue diseases (systemic lupus erythematosus, dermatomyositis and scleroderma)
Very large breast volume
Previous thoracic irradiation
Genetic predisposition to breast cancer
The patient’s ability to access a radiotherapy centre in relation to their general physical and psychological performance status and their logistical situation should be assessed before conservative surgery is planned. It should be kept in mind that some neurological or orthopaedic conditions such as severe upper limb functional limitations or neurodegenerative disorders like Parkinson’s disease can compromise the setup and ability to safely deliver the radiation treatment.
Specific contraindications to radiation treatment are traditionally divided into absolute and relative.
Pregnancy is an absolute contraindication to breast radiotherapy for its teratogenic risk (abortion, malformations, growth retardation and radiation-induced cancers). Before starting treatment, it is mandatory to ensure that women of childbearing age are not pregnant, and pregnancy must be prevented during the radiotherapy course. When a breast cancer diagnosis occurs during pregnancy, conservative surgery may be considered, and radiotherapy is usually postponed until after delivery, although the delay of the commencement of WBI might have some impact on efficacy. However, the possibility of receiving chemotherapy during pregnancy, as of the end of first trimester, may play a role on local control (see ► Chap. 42) [19].
Patients’ inability to maintain a stable treatment position (advanced Parkinson’s disease, advanced essential tremor, etc.) is an absolute contraindication because it prevents the correct execution of radiation treatment.
Li-Fraumeni syndrome is an absolute contraindication because, in these patients, ionizing radiation exposure increases the incidence of second malignancies.
Relative contraindications are shown below:
Connective tissue diseases involving the skin such as systemic lupus erythematosus, dermatomyositis and scleroderma, if quiescent, represent a relative contraindication and, if active, might be an absolute contraindication because of the amplification of reported toxicity. Rheumatoid arthritis is not considered a contraindication to radiation therapy. Moreover, new radiotherapy approaches, such as partial-breast irradiation, which reduces the irradiated volume, could be promising to improve the feasibility and tolerability of radiotherapy in patients with connective tissue diseases and will probably help to overcome the unresolved concerns about radiotherapy indications for patients with connective tissue diseases [20].
Severe lung disease with impaired respiratory capacity [21].
Severe cardiac disease for left-sided breast cancers [22].
The irradiation of very large breasts can be difficult due to the difficulty in reproducing the setup of dose delivery and the inhomogeneity of dose distribution, with a possible negative impact on post-treatment cosmesis. In these particular situations, radiation in the prone position or lateral decubitus position or, in selected patients, accelerated partial-breast irradiation (APBI) might be considered [23].
Previous thoracic irradiation, although not an absolute contraindication, should be assessed with caution and may be considered for PMRT or treatment of recurrent disease. The previous radiotherapy details (technique, volumes, dose and fractionation) and the interval between previous irradiation and the new planned treatment must be carefully evaluated before re-irradiation is undertaken. In case of re-irradiation, lower doses and smaller volumes are employed in order to limit normal tissue injury [24].
Women with a known genetic predisposition to develop cancers should be handled with care because of the increased risk of radiation-induced cancers (e.g. carriers of germline mutations in genes involved in the DNA damage repair pathway) [25].
The involvement of the multidisciplinary team evaluating the feasibility of radiotherapy is crucial in the assessment of the appropriateness of BCT for each individual patient.
39.3 Adjuvant Radiation Therapy After Breast-Conserving Surgery
39.3.1 Radiotherapy to the Whole Breast and Tumour Bed (Boost)
Radiation therapy to the whole breast following BCS is widely accepted as a standard of care for women with early-stage invasive breast cancer.
Long-term results from numerous randomized controlled trials have confirmed overall survival equivalence between BCT and mastectomy for early-stage invasive breast cancer (◘ Table 39.2).
Table 39.2
Overall survival from randomized controlled trials comparing breast-conserving therapy with mastectomy
Study and period of accrual | Pts (n) | Follow-up (years) | Treatment Type | Pts (n) | Overall survival |
|---|---|---|---|---|---|
Veronesi et al. [1] (National Cancer Institute of Milano) 1973–80 | 701 | 20 (median) | BCT | 352 | 59% at 20 years |
RM | 349 | 59% at 20 years (p = 1.0) | |||
Fisher et al. [2] (NSABP B-06) 1976–84 | 1851 | 20 (mean) | BCT | 628 | 46% at 20 years |
TM | 589 | 47% at 20 years (p = 0.57) | |||
Poggi et al. [3] (U.S. National Cancer Institute) 1979–87 | 237 | 18.4 (median) | BCT | 121 | 54% at 20 years |
MRM | 116 | 58% at 20 years (p = 0.67) | |||
Arriagada et al. [4] (Institut Gustave–Roussy) 1972–79 | 179 | 22 (mean) | BCT | 88 | 65% at 10 years |
MRM | 91 | 67% at 10 years (p = 0.16) | |||
Blichert–Toft et al. [5] (DBCG-82TM) 1983–89 | 731 | 19.6 (median) | BCT | 367 | 57.8% at 20 years |
MRM | 364 | 50.6% at 20 years (p = 0.20) | |||
Litière et al. [6] (EORTC 10801) 1980–86 | 868 | 13.4 (median) | BCT | 448 | 51.6% at 15 years |
MRM | 420 | 53.6% at 15 years (p = 0.225) |
In 1995, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis showed that in the nine trials of mastectomy versus BCS plus radiotherapy, there was no apparent difference in overall mortality (22.9% versus 22.9%) and local recurrences (6.2% with mastectomy as compared with 5.9% with breast conservation) [26]. Although these trials varied in inclusion criteria and in surgical technique and radiotherapeutic and systemic treatment protocols, they showed that BCT can be applied safely in patients with early breast cancer.
In modern practice, 5- and 10-year actuarial local recurrence rates of less than 5% are not uncommon with results near to 0% in some series, as in the ELIOT trial (0.4% in the external beam radiotherapy group after a medium follow-up of 5.8 years). The enormous improvement in surgery and radiotherapy techniques and systemic therapy with more effective cytotoxic, endocrine and targeted treatments, and higher-quality surgical procedures with much more diligent attention to achieving clear margins, has undoubtedly contributed to this reduction in local failure rates after BCT when compared to the historic trials [27, 28].
In addition to the randomized trials comparing breast conservation with mastectomy, different randomized trials have compared conservative surgery alone with conservative surgery and radiation, in order to investigate whether radiation following BCS can be omitted. The majority of randomized trials have demonstrated that radiation therapy following BCS lowers the local relapse rates by at least half and is associated with acceptable toxicity and morbidity [29]. The impact of radiation following BCS on breast cancer mortality or overall survival, however, has been the subject of ongoing debate until very recently. This issue has been clarified by the EBCTCG in three large meta-analyses published in 1995, in 2005 and finally in 2011 [8, 11, 26].
In its recent quinquennial meta-analysis update published in 2011, the EBCTCG included 17 randomized studies comparing adjuvant radiotherapy versus no radiotherapy following BCS. A total of 10,801 patients with pT1–pT2 tumours were recorded, the majority of whom (n = 7287) were node negative, 1050 node positive and 2464 nodal status unknown. The analysis not only reported significant 10-year absolute risk reduction of any (loco-regional or distant) first recurrence by 15.7% with radiotherapy following BCS but showed also that radiotherapy reduced the breast cancer death rate by about one-sixth. Overall, radiotherapy reduced the 15-year risk of breast cancer death from 25.2% to 21.4% (absolute reduction 3.8%, 2p = 0.00005) [8].
Given the impact of radiation on both local control and breast cancer-specific survival, the standard of care for the majority of patients with invasive breast cancers undergoing BCS is the use of radiation following BCS, and the avoidance of radiation therapy outside the context of a clinical trial must be approached cautiously.
There are, however, subsets of patients who are at lower risk of recurrence, for whom the absolute benefit might be clinically not meaningful. A common and clinically relevant example of this is in women over age 70 with hormone receptor-positive tumours, where observation following BCS is considered an acceptable option. However, given these concerns, many clinicians and patients would only consider the avoidance of radiation or the use of APBI in the context of a clinical trial [8, 30] (see ► Chap. 44).
The tumour bed is the area at highest risk for tumour cell contamination, and a number of randomized trials have investigated the effect of an additional dose of radiation to the tumour bed (known as boost) after the administration of WBI.
The European Organisation for Research and Treatment of Cancer (EORTC) has undertaken the 22,881–10,882 (boost versus no boost) trial in order to clarify whether this strategy has any merit or adverse effects [10, 31, 32]. In 5318 patients with a microscopically clear resection margin after surgery to the primary tumour, an additional dose of RT to the tumour bed nearly halved the annual odds of local recurrence (hazard ratio, 0.59), with a 10-year actuarial rate of local recurrence of 10.2% in the «no boost» group and 6.2% in the «boost» group (p < 0.0001). In the latest publication of this trial, with a median follow-up of 17.2 years, ipsilateral breast tumour recurrence had increased in both treatment groups (possibly as a consequence of development of second primaries): 16.4% for the «no boost» group and 12% for the «boost» group. However, the relative benefit of boost for local control remained statistically significant with an HR for an ipsilateral breast tumour recurrence as a first event of 0.65 (p < 0,0001).
Overall, boost did not improve overall survival which was 81.7% for both arms after 10 years of follow-up and 61.1% in the «no boost» group versus 59.7% in the «boost» group after 20 years; the failure of improved local control to increase overall survival has been hypothesized by the authors to derive from successful salvage mastectomy treatment for these breast recurrences.
Boost RT is associated with an increase in breast fibrosis; although not significant at 5 years, at 10- or 20-year follow-up, the extra boost dose has been shown to increase rates of moderate or severe fibrosis from 13% to 28% and from 15% to 30.4%, respectively. For this complication, the boost dose was not the sole factor that affected the cosmetic outcome negatively (other factors included the location of the primary tumour in the lower quadrants, the volume of breast tissue excised, administration of systemic therapy, breast infection or hematoma in the postoperative period and clinical T2 stage).
As early analyses found the largest clinical benefit from boost in younger patients (40 years old or younger), the authors tried to determine whether the effect of an additional radiation dose depended on age. The 2015 analysis confirmed that patients’ age was strongly correlated with the absolute risk of ipsilateral breast tumour recurrence: 20-year cumulative incidence ranged from 34.5% for patients 35 years or younger to 11.1% for patients older than 60 years, and the reduction of risk by giving a boost dose was significant for the younger age groups (for age ≤ 40 years, p = 0.003 and, for age 41–50 years, p = 0.007) [10, 31, 32].
Based on this landmark phase III trial, administration of a boost dose following WBI is strongly indicated especially if patients have high-risk factors for recurrence, such as age < 50 years old, pathologically involved ALN, lympho-vascular invasion and/or a close or positive resection margin. For the older women, its routine use is less clear, but, in patients over 60 years of age with small, node-negative, hormone receptor-positive tumours, omission of a boost may be reasonable [10, 33].
39.3.2 Radiotherapy to the Whole Breast, Tumour Bed and Regional Nodes
Regional node irradiation (RNI) should be considered in the following circumstances:
In cases with more than three ALN metastases, it is recommended.
In the case of one to three positive ALNs, it should be considered, especially when additional risk factors are present.
RNI includes the infra- and supraclavicular region and any part of axillary bed at risk. A number of radiotherapy centres include the internal mammary chain, irrespective of known neoplastic involvement. In 2016 the NCCN guidelines were extended to include the elective irradiation of internal mammary nodes, in light of recent publications.
Supported by similarities in tumour biology, most of the data available on RNI in patients treated with BCS were extrapolated from studies of patients undergoing mastectomy, in particular from three remarkable randomized clinical trials conducted by Overgaard and Ragaz. Furthermore, the historical recommendation to treat only in cases with more than three positive nodes was made despite the fact that these trials showed a benefit in survival for all node-positive patients [34–36].
Vicini and colleagues first demonstrated a statistically significant reduction in the rate of regional nodal failure in patients with more than three positive lymph nodes treated with BCT and RNI. The authors stated that, combining their study with the available literature, the data were «sufficient to support the inclusion of at least the supraclavicular fossa and level III ALN in patients with four or more positive nodes because of a statistically significant reduction in the rate of regional nodal failure with a relatively mild increase in toxicity» [37].
In 2011, the previously mentioned meta-analysis from the EBCTCG reported a 10-year improvement in the rate of any first recurrence of 21.7% in patients with four or more positive nodes who received adjuvant radiation therapy after BCS [8].
Currently RNI, in cases with more than three positive nodes, is recommended by all national and international guidelines [18, 38, 39].
However, some authors suggest that RNI, in particular RT to the supraclavicular fossa, even in patients with more than three positive nodes, cannot be routinely justified if the intent is to improve survival [40].
The need for RNI in cases of one to three positive nodes is currently an issue of intense debate.
Many studies published in recent years, mostly retrospective, have investigated this question. Despite the heterogeneity of these trials in the number of patients, analysis period, adjuvant systemic therapy regimes and radiotherapy volumes, these studies all emphasized the importance of different risk factors for local relapse, nodal relapse and distant metastases such as young age, tumour size, negative hormonal receptors, extensive intraductal component, high grade and nodal ratio >20–25%. So, in the presence of two or more of these risk factors, RNI should be considered [41–43].
Ideally, treatment recommendations should be made on the basis of well-designed phase III clinical trials. To date, the MA.20 and EORTC 22922/10925, two large randomized clinical trials with 85% and 43.1% of included patients having one to three positive ALNs, respectively, have been published as full papers. Both studies have stressed the need of RNI after BCS regardless of the number of positive lymph nodes, but they have not demonstrated any significant impact on OS from RNI.
The sites of nodes needed to be treated with the intent of improving OS and DFS remain unknown (the EORTC trial included internal mammary and medial supraclavicular lymph nodes; in the MA.20 trial, the internal mammary lymph nodes with the supraclavicular and ALNs were irradiated) [44, 45].
However, they both showed that RNI improves DFS, and distant DFS, without adding substantial toxicity; the EORTC trial also reported a small but significant benefit with respect to death from breast cancer (◘ Table 39.3).
Table 39.3
Results from MA.20, EORTC 22922/10925 and French trials
MA.20 [44] (median follow-up = 9.5 years) | EORTC 22922/10925^ [45] (median follow-up = 10.9 years) | French trial [63] (median follow-up = 11.3 years) | ||||
|---|---|---|---|---|---|---|
WBI (916 pts) | WBI + RNI (916 pts) | WBI (2002 pts) | WBI + RNI (2002 pts) | PMRT (662 pts) | PMRT + RNI (672 pts) | |
DFS | 77% | 82% p = 0.01a | 69.1% | 72.1% p = 0.04a | 49.9% | 53.2% p = 0.35 |
Isolated LR DFS | 92.2% | 95.2% p = 0.09a | – | – | – | – |
Distant DFS | 82.4% | 86.3% p = 0.03a | 75% | 78% p = 0.02a | – | – |
OS | 81.8% | 82.8% p = 0.38 | 80.7% | 82.3% p = 0.06 | 59.3% | 62.6% p = 0.8 |
BC mortality | 12.3% | 10.3% p = 0.11 | 14.4% | 12.5% p = 0.02a | – | – |
Due to this ongoing uncertainty, RNI in cases with one to three positive ALNs should be discussed in a multidisciplinary team meeting and then discussed with the patient.
The indication for radiotherapy to the axilla is generally restricted to patients with recurrent disease, or if ALNs are clinically involved and no axillary dissection is possible or planned, despite the EORTC 10981–22,023 AMAROS trial, where patients with clinical T1–T2 N0 primary breast cancer were randomly assigned to axillary radiotherapy or axillary lymph node dissection, it showed that, for patients with a positive sentinel lymph node, either modality provides excellent and comparable axillary control. Moreover, the rates of lymphoedema were lower in patients treated with axillary radiotherapy than in those treated with axillary lymph node dissection [46].
39.4 Adjuvant Radiation Therapy After Mastectomy
After mastectomy for early breast cancer, radiation therapy for clinical stages I–II and T3N1M0 breast cancer depends on tumour size, ALN involvement and the state of the surgical margins. General indications for post-mastectomy radiation therapy (PMRT) are listed below:
Tumour >5 cm
Four or more positive ALNs (PMRT is mandatory)
One to three ALNs (PMRT is recommended)
Positive surgical margins when further surgery is not possible
Chest wall/skin infiltration (T4a, T4b, T4c)
Inflammatory cancer (T4d)
Pectoral muscle invasion
Generally, radiation therapy to the chest wall only is recommended in the case of T3 N0 tumours, positive surgical margins or pectoral muscle invasion; in the remaining situations, PMRT includes chest wall and regional lymph nodes.
39.4.1 Radiation Therapy to the Chest Wall
There is strong evidence that tumour size greater than 5 cm, as a sole negative feature, is a risk factor for loco-regional recurrence (LRR), so chest wall irradiation is traditionally recommended for these patients, but there is controversy concerning the real need for PMRT for pT3N0M0 breast cancer due to low recurrence rates after a median follow-up time of 10 years or more [12, 47, 48].
A comprehensive review of studies including T3 N0 patients was performed indicating the main risk factors and risk categories for local relapse. These include lympho-vascular invasion, blood vessel invasion, positive lymph node ratio > 20%, close resection margins <3 mm, grade 3, young age/premenopausal status, extracapsular nodal invasion, negative hormone receptor status and invasive lobular cancer. These high-risk factors help to identify T3 N0 patients who will benefit from PMRT [49].
Positive and close surgical margin status is generally believed to be associated with high LRR risks, but there are few available studies examining this issue in the post-mastectomy setting and even fewer analyses focusing on the specific role of PMRT in these patients [50, 51]. In spite of this, chest wall irradiation is recommended in patients with positive surgical margins (if there is no option for further surgery) and should be considered for women with a close resection margin and one or more additional risk factors (age ≤50 years, T2 tumour size, grade 3, lympho-vascular invasion, triple-negative disease) [18, 51, 52].
39.4.2 Loco-Regional Radiation Therapy (Chest Wall and Regional Nodes)
Regarding nodal status, historically PMRT to the chest wall and regional lymph nodes has been delivered in the case of four or more positive nodes at axillary dissection [47, 53–56]. The background for this indication was the general assumption that only patients with very high risk of LRR will benefit from postoperative radiotherapy in terms of survival and, as expressed in the National Institutes of Health consensus report 2000, «….the high-risk group includes women with four or more positive nodes» [57].
Three well-conducted randomized trials have shown that, for node-positive breast cancer patients unselected for the number of lymph nodes involved, irradiation of the chest wall and regional lymph nodes after mastectomy and ALN dissection improves DFS and OS in addition to loco-regional control [34–36]. In the DBCG 82b trial (1708 high-risk premenopausal patients), the 10-year loco-regional recurrence rate was 9% in PMRT + chemotherapy patients in contrast to 32% in patients treated with chemotherapy alone, and the corresponding survival rates were 54% and 45%, respectively, in favour of PMRT + chemotherapy (p < 0.001). The DBCG 82c trial (1375 high-risk postmenopausal patients) showed a 10-year loco-regional recurrence rate of 8% in PMRT + tamoxifen patients compared to 35% in tamoxifen-only patients with the corresponding 10-year survival rates of 45% and 36%, respectively, in favour of PMRT + tamoxifen (p = 0.03). Remarkably, there has been an ongoing debate on whether or not PMRT should be given to patients with one to three positive ALN. In a subgroup analysis of the Danish trials, the addition of radiation therapy resulted in a substantial reduction in the 15-year LR failure rate from 27% to 4% (p < 0.001) and from 51% to 10% (p < 0.001), in patients with one to three versus four or more axillary positive nodes, respectively. The 15-year survival benefit after radiation therapy was equally pronounced in both groups (57% versus 48%, p = 0.03, and 21% versus 12%, p = 0.03). As PMRT improved the outcome to the same extent in the two groups, the authors suggested considering a «modification of the current guidelines for indications for post-mastectomy irradiation» [34, 35, 58]. Finally, Ragaz and colleagues (318 premenopausal high-risk patients, PMRT + CMF versus CMF without PMRT) observed that after a median follow-up of 15 years, there was a significant absolute improvement of 20% in local relapse free survival (p < 0.003) with a corresponding 8% improvement in survival rates (54% versus 46%, p = 0.07). The 20-year results of this trial confirmed previous analyses showing that the impact of radiation therapy for all survival outcomes in the subgroup with one to three nodes involved was similar to the subgroup with four or more nodes involved and both groups had a risk reduction of the same order of magnitude [36, 59].
The recently published update of the EBCTCG meta-analysis should bring the debate about patients with one to three positive ALN to an end, since it unequivocally demonstrated that these patients receive the same survival benefit from PMRT as patients with more than three involved lymph nodes. In 1314 patients with pN+ (one to three nodes), the risk for local recurrence at 10 years was significantly reduced from 20.3% to 3.8%, and breast cancer mortality decreased significantly by 7.9% after 20 years; in comparison, the risk for local recurrence in 1772 patients with pN+ (four or more) at 10 years was significantly reduced from 32.1% to 13.0%, and breast cancer mortality was also reduced by 9.3% at 20 years. Importantly, after mastectomy and axillary dissection, radiotherapy reduced both recurrence and breast cancer mortality in the women with one to three positive lymph nodes even when systemic therapy was given [13].
Despite these results, routine use of PMRT in the case of less than four ALNs is not yet the standard of care, and consequently its general use in patients with tumours <5 cm is based on the presence of other prognostic risk factors (age < 40–45, tumour diameter > 3.5 cm, ER and PgR negativity, lympho-vascular invasion, grade 3 or a positive lymph node ratio > 20%) [60–62].
The results of the Medical Research Council Selective Use of Postoperative Radiotherapy After Mastectomy (SUPREMO) trial, a randomized phase III trial assessing the role of irradiation in women with intermediate-risk breast cancer following mastectomy, will help determine the real impact of PMRT in this subgroup of patients in terms of loco-regional control and overall survival, but it will take years before a definitive answer is obtained [63].
The role of irradiation of internal mammary nodes (IMNs) remains unclear as a French trial failed to demonstrate a survival benefit for IMN irradiation in patients with positive axillary nodes (pN+) or central/medial tumours with or without pN+, and the EORTC 22922/10925 (23.9% of the entire population were treated with mastectomy) cannot determine whether internal mammary irradiation or medial supraclavicular irradiation contributed more to the outcome [64].
On the other hand, the results from the Danish population-based study in which patients with left-sided node-positive breast cancer underwent only medial supraclavicular irradiation, whereas patients with right-sided node-positive breast cancer underwent both internal mammary and medial supraclavicular irradiation, support the role of including the internal mammary chain in the success of regional nodal radiation therapy (the study included patients treated both with lumpectomy and mastectomy) with a benefit on overall survival [65].
In the meantime, the use of PMRT in patients with early-stage breast cancer should be considered a valuable option, and its recommendation should be individualized according to patient and tumour features.
In ◘ Table 39.4 general indications for radiotherapy in early breast cancer are summarized.
Table 39.4
Summaries of general indications for radiotherapy in early breast cancer (see the text for more details)
Indications | Notes |
|---|---|
After breast-conserving surgery (radiation therapy to the residual mammary parenchyma ± regional nodes) | Should be performed in almost all subset No subsets are identified which can safely avoid radiation therapy Benefit is minimal in older women with T1 N0 ER+ breast cancer Add radiation therapy on regional nodes in the case of more than three positive axillary nodes (it should be considered in the case of 1–3 positive axillary nodes) |
After mastectomy, positive axillary nodes (radiation therapy to chest wall and regional nodes) | Mandatory in the presence of four or more positive axillary lymph nodes Recommended for patients with one to three axillary lymph nodes involved |
After mastectomy, negative axillary nodes (radiation therapy to chest wall ± regional nodes) | Recommended for tumour size >5 cm Recommended for positive surgical margins To consider in the case of close margins and other risk factors |
39.5 Radiation Treatment Planning and Delivery
It is important to individualize radiation therapy delivery, and CT-based treatment planning is mandatory to delineate target volumes and adjacent organs at risk.
According to the indications reported in the previous paragraphs, volumes of interest of radiation treatments are listed below:
After BCS: the target includes the residual mammary gland (the skin should not be included unless it is affected by the disease), the site of the primary tumour (boost) and drain sites when indicated.
After mastectomy: the target includes the ipsilateral chest wall (skin and rib plane), mastectomy scar and drain sites when indicated.
Supraclavicular and infraclavicular nodes: the term supraclavicular is synonymous with inferior deep cervical nodes according to the AJCC classification of the head and neck region. The term infraclavicular nodes is synonymous with apical or level III axillary nodes.
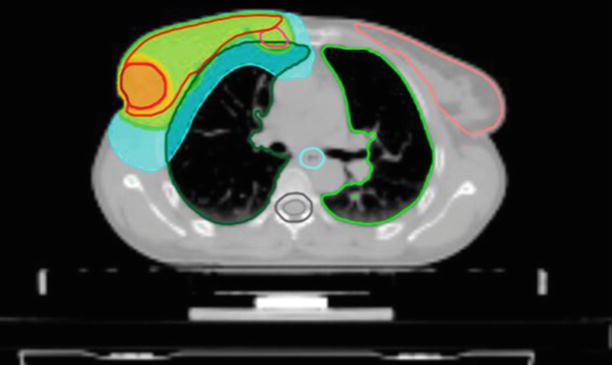
Internal mammary nodes: the target volume should be restricted to the ipsilateral side and usually should not extend below the third or fourth intercostal space (◘ Fig. 39.1).
Axillary nodes (ALNs): the ALNs are divided into level I (low axilla) and level II (mid-axilla). Level III (apical axilla) corresponds to the infraclavicular nodes.

Fig. 39.1
Transverse view of a treatment plan for whole-breast RT with simultaneous integrated boost and internal mammary chain irradiation. The green colour corresponds to 95% of the prescribed dose to the breast and internal mammary nodes, the orange colour to 95% of the boost dose, the blue colour to 25% of the boost dose (Tomotherapy® Treatment Planning System)
For node identification, the corresponding arteries and veins can be used as a surrogate for nodal location (as the nodes themselves are not usually visible on planning imaging). Accurate knowledge of radiological anatomy is required for delineation of targets mentioned above; however, contouring is a process prone to errors and inter- and intra-operator variability. In particular, the tumour bed is subject to topographical uncertainties; consequently, many authors recommend the use of surgical clips to mark the lumpectomy site [66]. Moreover, several anatomically based guidelines have been published for definition of the planning target volume and organs at risk like the heart and lung [39, 67–69].
For planning and treatment, the patients lie in the supine position with one or both arms in abduction (>90°) on a breast board. Prone positioning may be tried to further reduce the dose to adjacent normal tissues.
Different techniques can be used to deliver radiation treatment: homogeneity in dose distribution is usually achieved by a conformally shaped tangential wedge field technique, mostly with photons at energies of 4–8 MV (◘ Fig. 39.2). For chest wall irradiation, single electron beam field of appropriate energy may be an alternative allowing for better sparing of underlying tissues. Intensity-modulated radiotherapy (IMRT) allows for better dose conformation and reduction of the dose to the surrounding organs. This may allow for wider implementation of hypofractionated schedules or a concomitant boost approach (see below). For larger breasts or for patients with difficult anatomical variants (i.e., funnel chest, pectus excavatum), IMRT or the use of active breathing control may be appropriate to fulfill the minimum homogeneity criteria and to spare radiation to the lungs and to cardiac structures (for left-sided tumours), respectively [70, 71].
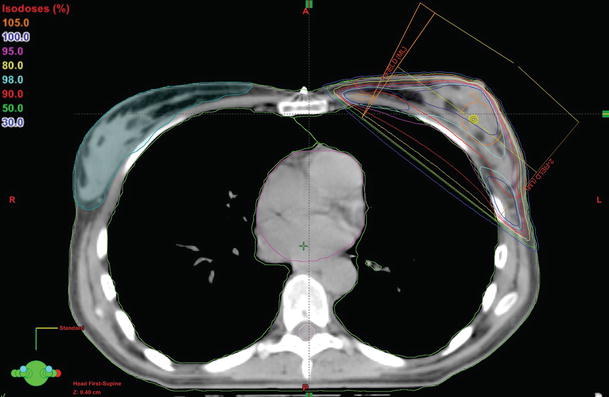

Fig. 39.2
Transverse view of a treatment plan for whole-breast RT with a conformally shaped tangential fields techniques (relation between colours and prescribed dose is shown on the left of the figure; ML medial-lateral, LM lateral-medial)
The dose-volume histogram (DVH) is a graphical representation of the dose that is received by normal tissues and target volumes within a radiation therapy plan (◘ Fig. 39.3).
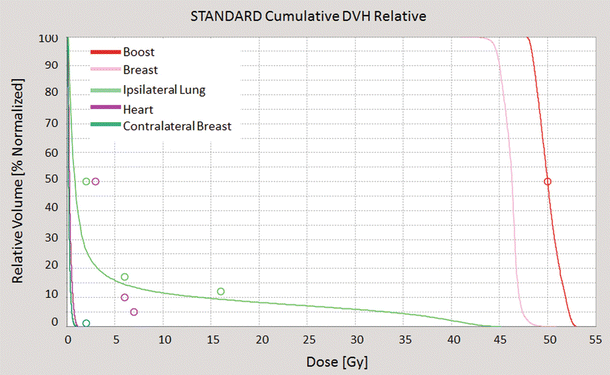

Fig. 39.3
An example of a dose-volume histogram: dose distribution (horizontal axis) is related to organ volume (vertical axis); every structure corresponds to a different colour (red, boost; pink, breast; green, ipsilateral lung; violet, heart; dark green, contralateral breast) (Tomotherapy® Treatment Planning System)
Correct treatment delivery has to be documented with verification of daily setup consistency. Several imaging modalities are available (electronic portal imaging device, cone beam CT, etc.). The choice of modality of imaging, frequency (daily, weekly, etc.) and working mode (online/offline verification) is not yet standardized and depends on the technique and institutional workflow [72].
Four randomized clinical trials have investigated hypofractionated WBI schedules (39–42.9 Gy in single fractions of 2.6–3.3 Gy) compared to standard 50 Gy in single fractions of 2 Gy. These studies with 10-year follow-up reported that local tumour control and breast cosmesis were similar with a hypofractionated regimen compared with 50 Gy in 25 fractions over 5 weeks (◘ Table 39.5) [16, 17, 73, 74].
Table 39.5
Characteristics and outcomes of randomized hypofractionation trials
Dose comparisons (Gy/fraction/wk) | Number of patients | 10-year local control (%) | 10-year cosmesis (% with good/excellent or «no event») | |
|---|---|---|---|---|
Owen [73] (RMH/GOC trial) | ||||
Control | 50/25/5 | 470 | 87.0 | 28.8 |
Arm 1 | 39/13/5 | 466 | 85.2 | 42 |
Arm 2 | 42.9/13/5 | 474 | 90.4 | 25.6 |
Bentzen, 2008 [16] (START trial A) | ||||
Control | 50/25/5 | 749 | 92.6 | 72.9 |
Arm 1 | 39/13/5 | 737 | 91.2 | 78.4 |
Arm 2 | 41.6/13/5 | 750 | 93.7 | 71.8 |
Bentzen, 2008 [17] (START trial B) | ||||
Control | 50/25/5 | 1105 | 94.5 | 68.8 |
Arm 1 | 40/15/3 | 1110 | 95.7 | 73.8 |
Whelan [74] (Canadian trial) | ||||
Control | 50/25/5 | 612 | 93.0 | 71.3 |
Arm 1 | 42.5/16/3.5 | 622 | 94.0 | 69.8 |
Based on convenience and data from trials, in many countries, short-course radiation therapy is now considered the «gold standard» for adjuvant breast cancer radiotherapy [75, 76].
The conventional post-mastectomy RT dose is 45–50.4 Gy, given in 1.8–2.0 Gy fractions. Although there is no high-quality evidence that hypofractionation has similar efficacy and safety as conventionally fractionated radiation treatment for early breast cancer, the large British Columbia randomized controlled trial demonstrated an overall survival benefit of PMRT with a hypofractionated schedule (37.5 Gy in 16 fractions), and retrospective studies showed that short courses of radiotherapy are as effective as conventional radiotherapy in the post-mastectomy setting [36, 77]. Moreover, a small percentage of post-mastectomy patients were included in the START A and B trials. A randomized controlled trial would be necessary to more completely assess the acute and long-term toxicity of post-mastectomy hypofractionated radiotherapy compared with standard fractionation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







