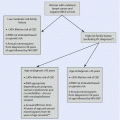
Fig. 24.1
Algorithm summarizing standard axillary management
24.2.2 Preoperatively Detected Axillary Metastases
A direct AC without SLNB remains the standard of care in clinically node-positive breast cancer patients, with preoperatively detected axillary lymph node metastases [5, 7]. In these patients, AC provides accurate knowledge of the total number of metastatic lymph nodes, which is an important prognostic indicator, although it may not have much value in adjuvant therapy decision-making [8].
The high sensitivity of modern preoperative axillary ultrasound may detect single axillary metastases even with no suspicious nodes on palpation, which poses an obvious overlap with the patient population with SLNB-detected axillary macrometastasis. This overlap and inconsistency in treatment protocols (see ► Chap. 23 on SLNB) is the subject of ongoing research. In patients with preoperatively detected high-volume tumour burden in the axilla, primary systemic chemotherapy is commonly considered.
24.2.3 Sentinel Node Metastasis
The matter of the tumour-positive sentinel node is covered in detail in ► Chap. 23. In summary, axillary clearance is not indicated in cases with tumour-negative sentinel nodes. Similarly, neither isolated tumour cells nor micrometastasis in the sentinel node is usually considered an indication for axillary clearance [5, 9]. However, in cases of macrometastatic sentinel node(s), there is considerable variation in treatment protocols between countries and centres with many centres adopting individualized treatment algorithms based on patient-specific characteristics and multidisciplinary team evaluations.
A number of risk prediction tools and nomograms have been published to evaluate the patient-specific risk of additional metastases or N2 disease risk after tumour-positive sentinel node findings [10–12]. Many of these prediction tools are published as online calculators that provide the patient-specific risk as a risk percentage. Such aids may be used in the decision-making process for AC after finding a metastatic sentinel node. Nonetheless, there is no consensus on clinically applicable risk thresholds for non-sentinel node metastases or N2 disease that would indicate the need for AC or axillary radiotherapy. Furthermore, the accuracy and performance of a specific prediction model should be assessed and validated for use in each centre before adoption into clinical practice.
24.3 Anatomy of the Axilla
The axillary lymph node basin is a triangular space bordered laterally by the latissimus dorsi muscle, medially by the serratus anterior muscle and the thoracic wall, cranially by the axillary vein, anteriorly by the pectoralis major and minor muscles and posteriorly by the latissimus dorsi, teres major and subscapularis muscles.
The axillary lymph nodes are divided into three anatomical levels according to Berg [13]: Level I lymph nodes are located lateral to the pectoralis minor muscle, whereas level II lymph nodes are posterior, and level III lymph nodes are medial to the pectoralis minor muscle (◘ Fig. 24.2).
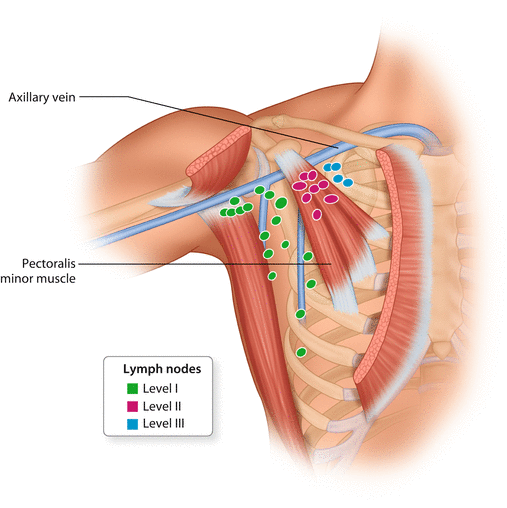

Fig. 24.2
Axillary lymph node levels I–III, according to Berg
The axillary lymph nodes are surrounded by axillary fat, which is also crossed by a number of nerves and vascular structures. The long thoracic nerve is the motor nerve of the serratus anterior muscle. It lies deep in the axilla running in a cranio-caudal direction along the serratus anterior muscle, lateral to the chest wall. Damage to the long thoracic nerve may cause paresis to the serratus anterior muscle, which can be clinically manifest by ‘winging of the scapula’. The thoracodorsal bundle includes the motor nerve, artery and vein which supply the latissimus dorsi muscle. The thoracodorsal bundle crosses the axillary fat lateral to the long thoracic nerve. The vein of the bundle intersects with the axillary vein at the cranial border of the axilla, whereas the nerve and the artery dive posterior to the axillary vein. The intercostobrachial nerve(s) is a sensory nerve innervating a variable area of the skin of the dorsum of the upper arm. Generally the nerve(s) branches from the intercostal nerves and runs through the serratus anterior muscle, crosses the level I axillary fat parallel but caudally to the axillary vein and enters the arm. However, there is substantial anatomical variation in the course and branching of this nerve [14–16]. Damage or transection of the intercostobrachial nerve causes variable sensory changes to the area it innervates. The lateral thoracic vein and artery run along the serratus anterior muscle, anteriorly to the long thoracic nerve. The medial pectoral pedicle comprises the medial pectoral nerve and accompanying vascular vessels. It is located at the lateral border of the pectoralis minor muscle. The medial pectoral nerve innervates both pectoralis minor and part of the pectoralis major muscles. Damage to the medial pectoral pedicle therefore may cause paresis of the pectoralis muscles [17].
24.4 Surgical Technique of Axillary Clearance
When axillary clearance is indicated in breast cancer patients, a dissection of Berg levels I and II should be conducted as routine. If clinically suspicious nodes are palpable medial to level II, level III should also be cleared, although this is often done as routine. Generally, if axillary clearance is performed, all clinically suspicious nodes should be removed, and sometimes neurovascular structures may have to be sacrificed in order to achieve radical removal of all cancerous tissue.
There is undoubtedly abundant variation in the surgical technique in dissecting the axillary lymph nodes. The dissection can be performed either via the same incision as the breast operation, such as mastectomy or lateral breast-conserving surgery, or through a separate incision to the axilla. The dissection is typically begun from the caudal part of the axilla, proceeding up towards the axillary vein and then continuing medially to the Berg level II. The long thoracic nerve and the thoracodorsal bundle should be identified and carefully separated from the axillary fat. The dissection may then follow the route of the thoracodorsal bundle to the axillary vein.
There is currently no consensus on the optimal handling of the intercostobrachial nerve(s) during AC [14–16, 18–20] and whether to preserve or perform a planned clean transection of the nerve(s) thus remains unclear. If the nerve(s) is cut, it should be performed sharply close to the thoracic wall medially and at the level of subcutaneous fat laterally.
The AC is continued by dissection of the axillary fat from the lateral border of the pectoralis muscles, and the medial pectoral pedicle is identified and preserved. The pectoralis muscles are then retracted anteriorly to facilitate the dissection of levels II and III by following the axillary vein medially. The lateral thoracic vein unites with the axillary vein at the border of levels I and II, and the lateral thoracic vessels may be spared if feasible. In special cases with challenging dissection of the level III nodes through the standard axillary approach, a direct transpectoral approach may be considered. In this approach the pectoralis major muscle is split anteriorly, and the pectoralis minor is retracted laterally, with the level III axillary fat accessed directly from the anterior direction [21].
There is considerable individual variation in the axillary anatomy regarding the neurovascular structures [16, 20, 22]. The axillary arch or Langer’s arch is perhaps the most important anatomical variation concerning the AC [23]. It is an aberrant muscular slip of varying dimension, which typically arises from the latissimus dorsi muscle, crosses the axilla anteriorly to the axillary fat and connects with the pectoralis muscles. The axillary arch may need to be divided in order to facilitate axillary clearance.
After the axillary clearance, a careful palpation of the entire axilla must be performed, and all remaining suspicious nodes should be removed. In particular the interpectoral space, i.e. the space between the pectoralis minor and major muscles, should be palpated, and palpable nodes should be removed. The space between the thoracodorsal bundle and the long thoracic nerve and especially the lateral aspect of the junction of the thoracodorsal vein and axillary vein are typical locations for retained lymph node metastasis and subsequent lymph node recurrences (◘ Fig. 24.3).
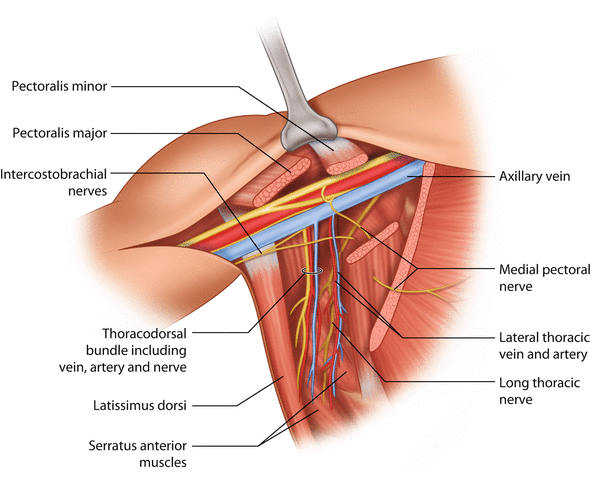

Fig. 24.3
Key anatomical structures of the axilla
Finally, meticulous haemostasis is performed, and closed-suction drainage may be left in the axilla, but this is a controversial subject at present. Seroma formation is common after axillary clearance, and a number of studies have looked at measures to reduce seroma incidence. Surgical obliteration of the dead space by quilting sutures seems to reduce seroma incidence both in the axilla and in the mastectomy area [24–26]. A 2013 Cochrane systematic review and meta-analysis concluded that based on seven randomized trials, there is limited quality evidence that wound drainage reduces seroma formation and the number of postoperative seroma aspirations [27]. A subsequent randomized trial of 596 breast cancer patients contested this finding by concluding that drainage did not reduce symptomatic seroma formation or interventions to treat seromas after axillary clearance [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree