Fig. 51.1
MRI multiple cerebral breast cancer metastases. T1 plus gadolinium contrast scan shows several variable sized lesions in the cerebral hemispheres, cerebellum and brain stem. The midbrain lesion (sagittal plane) shows a pineal region metastasis threatening CSF pathway drainage and incipient hydrocephalus as a further complication of these lesions and a cause of headache and balance deterioration. A ventriculoperitoneal shunt may be indicated. These cases are best managed with WBRT
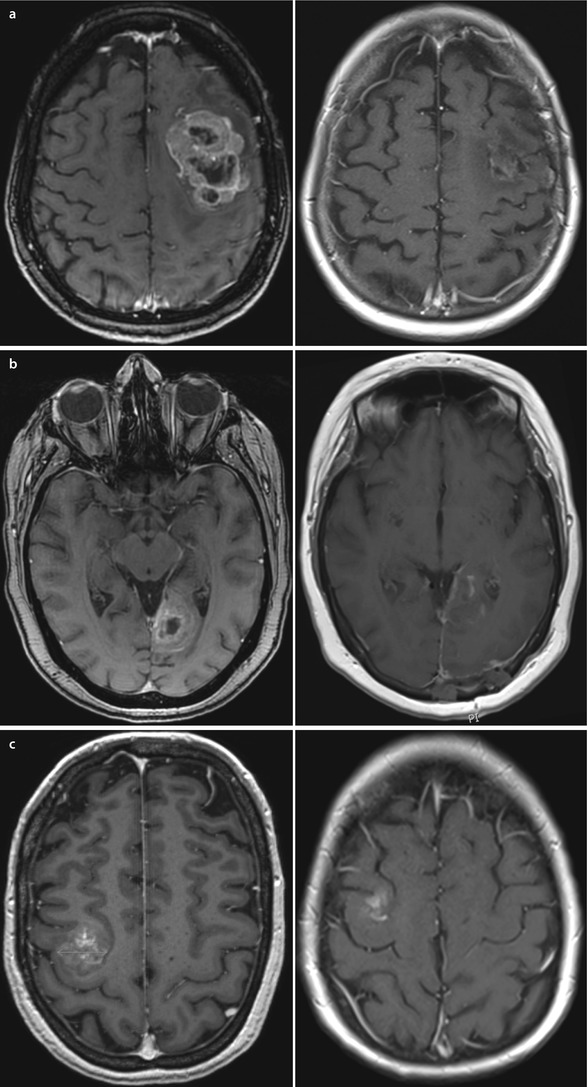
Fig. 51.2a–c
a T1 axial MRI with contrast. Large left frontal metastasis – unsuitable for any RT and best managed by craniotomy and surgical removal, as demonstrated b T1 axial MRI with contrast. 3+ cm midline left occipital lesion with dural base with immediate postoperative view showing good excision of mass lesion c T1 axial MRI with contrast. 2.7 cm lesion involving motor strip on the right side. Surgical excision involved preoperative magnetic stimulation to mark out active motor strip components and preoperative electrocortigraphy (mapping) to define approach to removal of this lesion. Immediate postoperative scans confirm removal with minimal haematoma in resection bed and no deficit
Histologically breast metastases resemble the primary lesion. Occasionally frozen section will be indicated where diagnosis is in question. However it is the presence of known primary disease that makes the likelihood of a new cerebral lesion being secondary disease greater than 90% [10]. Immunohistochemistry can identify the primary origin: Breast tumours are positive for cytokeratin 7 as are lung, gastrointestinal tract (GIT) and ovary. Most lung tumours however are positive for TTK unlike breast, and cytokeratin 20 is positive in breast and lower GIT. The presence of oestrogen and progesterone receptors might support a diagnosis but can be present in secondaries from other sites. Gross cystic disease fluid protein (GCDFP15) expression is a useful positive marker for breast origin where present and lack of E cadherin expression is useful particularly in lobular carcinomas.
51.4 Clinical and Imaging Features
Two thirds of brain metastases are symptomatic during the patient’s lifetime. The signs and symptoms are the same as for any other slowly expanding intracranial lesion ultimately resulting in raised intracranial pressure. There are three main symptoms: seizures, focal symptoms and signs and headaches.
Seizures
Up to 20% of patients with metastases will present with seizures. However around 50% will have seizures at some time in their illness. New onset seizures are better treated with early introduction of effective antiepileptic drugs (AEDs) such a Levetiracetam 250–500 mg bd rather than steroids, although the latter may be required where there is obvious evidence of cerebral oedema on T2-weighted MRI or CT scanning or additional indications of globally raised intracranial pressure. Seizures most commonly are focal in nature but may present acutely with a major convulsion. Prolonged seizures in patients with intracranial metastases is an emergency even with focal seizures, as ensuing brain swelling added to existing oedema can result in dramatic brain swelling and death.
Focal Symptoms and Signs
As well as focal seizures suggesting a locus for an intracranial lesion, progressive loss of function, hemiparesis and hemianopia can be useful indicators of the presence of an expanding lesion. Where the pattern reflects more than one metastasis, the clinical signs can help decide which is the most potentially harmful in terms of clinical impact. Hence the assessment of focal symptoms and signs may be confounded by suggesting more than one location where multiple lesions are present. Sudden focal deterioration can occur often suggestive of a «stroke», but CT/MRI scanning may indicate haemorrhage associated with a metastatic deposit.
Headache
This occurs in less than 50% of patients with intracranial metastases and classically reflects a later stage in the disease where the cumulative mass effect of the tumour or tumours and their associated oedema results in raised intracranial pressure. In these situations assessment for papilloedema as part of the examination is mandatory to assess the risk of acute deterioration. Headache due to raised intracranial pressure from tumour-related mass effect is a neurosurgical emergency, and advice should be sought immediately. The rapid introduction of intravenous or oral dexamethasone (with gastric protection) is advocated.
Headache may also occur where the lesions are attached to the dural surface towards the midline or in the middle fossa or to the falx itself. Probably local inflammation and associated direct trigeminal innervation are responsible (see ◘ Fig. 51.1).
Most importantly in patients with known primary or metastatic breast cancer, any patient with unexpected new neurological symptoms should be carefully screened and imaged for spread of disease to the CNS. For patients without a history or evidence of previous or coexisting cancer, the likelihood of a single brain lesion being a metastasis is less than 15%.
Immediate screening of patients with suspicious symptoms and signs is frequently performed with CT head scans. To enhance and expedite MDT decision-making where intracranial lesions are seen, MRI head scanning is essential, and in some tumour types, or where symptoms are suggestive of CNS involvement outside the cranium, e.g. thoracic pain, imaging should include the whole cranio-spinal axis. Commonly patients with isolated cranial lesions will need staging or screening with CT of the chest, abdomen and pelvis for the multidisciplinary team/tumour board (MDT) to be in a position to decide on action. Contrast-enhanced MRI is more specific and much more sensitive at showing up small or multiple lesions. It is also critical for determining lesions in the cerebellar or posterior fossa area where bone shadows make CT interpretation unreliable. MRI slice thickness tends to be finer, and hence the chance of visualising small lesions for both radiotherapy planning and monitoring is improved. Increasingly units offering access to both stereotactic radiosurgery (SRS) and surgery for these patients will request specific thin cut planning scans that can be used for image-directed surgery and/or fused with CT scans or alone for RT planning. With enhanced T1-weighted MRI scans, metastatic deposits show as discrete areas of increased signal intensity (white spots) with a tendency to a ringlike appearance as the size of the lesions increases. Not infrequently contrast penetration and retention is time- and dose-dependent, and where there is doubt about a lesion’s nature or presence, then the use of double-dose contrast can be useful. This latter approach is especially useful for SRS planning. Tumour-related oedema is often considerably greater than one might expect for the size of the lesion and is usually well seen on T1-weighted (decreased intensity) imaging but often more measurable and visible on T2 scans where the parenchymal «white signal» is seen. Cystic metastatic tumours may have a thicker wall than that seen in high-grade gliomas, but there is little to separate these from the appearances of an abscess cyst except the obvious clinical presentation. Tumours adherent to the dura or falx need careful anatomical review to clarify the local extent on this surface for both surgical and SRS planning. It is not possible to resolve the degree of local invasion of the tumour deposits from either very clear or fuzzy margins on MRI imaging. There is some suggestion paradoxically that sharp imaging margins may need more careful examination of adjacent vessels at surgery for tumour involvement.
Diffusion-weighted imaging may offer some help with deciding on the extent of additional resection required, but the data so far is anecdotal. MRI/ADC mapping may offer information on very early responses to treatment in residual lesions and hence may be a method for evaluating new (medical) therapies. Magnetic resonance spectroscopy (MRS) can be helpful in particular situations where the differentiation between glioma, abscess, lymphoma and metastasis is in question.
51.5 Approaches to Treatment
51.5.1 Medical
Patients with symptoms related to their brain metastases usually obtain both focal and global benefit from the early introduction of oral steroids. Dexamethasone is normally available in 0.5 and 2 mg tablets. Up to 16 mg per day in divided doses can be very effective and should be accompanied by a single morning dose of a gastric protectant such as lansoprazole 30 mg or omeprazole 10 mg. The neuro-beneficial effects come on within hours, but care is needed with diabetic patients that glucose levels do not rise unacceptably. Prolonged use of steroids will often cause a Cushingoid appearance and hence an additional burden on already debilitated patients. A few, mostly older, patients can develop steroid-related psychological changes, even psychoses that can be misconstrued as tumour-related deterioration and exacerbated by dosing in the evenings which can lead to disturbed sleep patterns. With early control of symptoms with higher steroid doses, the dose should be reduced and titrated against symptoms to reduce or limit the risks of confounding steroid-induced proximal myopathy. The longer-term problems with steroid use are well known including hypothalamic-pituitary-adrenal axis suppression where patients have been taking steroids for more than 6 weeks and profound osteoporosis which can lead to vertebral bone pain from microfracturing as well as fractures from avascular necrosis in the hip and long bones. Careful management of steroid dosing is an important and essential part of these patients’ care. Every effort should be made to reduce steroids to the lowest dose required, and indeed one of the benefits of cranial surgery may be to help achieve this.
Concomitant medications may need review especially diabetic treatment for patients on steroids. It is useful if surgery is being considered to review the use of antiplatelet therapies including routine aspirin as this may delay access to surgery.
51.5.2 Definitive Therapy
The treatment of brain metastases is based on the selected use of surgical resection and/or radiation therapy. Determining how best to use these techniques depends firstly on the number, size and location of the lesions. Secondly the general health of the patient and the staging of their systemic disease and their neurological performance provide a crucial basis for decision-making and are strong indicators of the outcome from intervention. Thirdly it is necessary to consider the likely radiosensitivity of the lesions and their likely response to systemic therapies based on their molecular subtype (Her2 receptor expression, ER receptor expression, for example) and the bioavailability of suitably targeted agents in the brain. Consideration of these three main areas informs the MDT discussion and allows the team to direct approaches to patients with controlled primary disease with the objective of long-term cure/control of disease in the brain or more palliative control towards improvement in quality of life for patients with both extracranial and brain lesions. Graded prognostic assessments (GPA) may provide a basis for stratifying molecular-defined patient groups for further interventional studies, with Her2-positive patients harbouring cerebral metastases showing improved survival over Her2 negative [11].
51.6 Whole Brain Radiotherapy (WBRT)
The use of whole brain radiation therapy for the treatment of brain metastases offers a seemingly simple and non-invasive way of treating the entire brain and hence would appear ideally suited to treating multiple metastases, and there is good evidence that breast and lung cancers demonstrate relative radiosensitivity by comparison with melanoma or renal cell tumours.
For many patients the presence of multiple deposits with very small or apparently microscopic collections makes this the only really viable treatment that can be offered. However it is increasingly recognised that large volumes of normal brain are exposed to ionising radiation, and in situations where the expectation of good control or cure means that life expectancy is increased, then it might be questioned whether preservation of normal function may be threatened despite fractionated treatments. Brain tissue sensitivity to RT is variable and depends on the total dose, fraction size, volume and dosing interval. Acute side effects from a multiple fraction course to the brain include hair loss, headaches, nausea, otitis media and very commonly lethargy with all-pervading tiredness and on occasion acute skin irritation and desquamation. The symptoms of fatigue can take several weeks to abate after the RT course, and patients may be left with this as their major chronic symptom. A further acute issue can be dramatic brain swelling that can require large (even very large) dose of steroids, and on occasion acute decompressive surgery may be considered appropriate. A number of patients see their lethargy develop into a «somnolence syndrome» characterised by a chronic debilitating fatigue [12]. Late effects are also well recognised including atrophic change in the brain with associated memory or cognitive deficits, radiation necrosis that can masquerade as a new or recurrent tumour mass, progressive white matter disease (leucoencephalopathy) and with mainly frontal RT, a dementing disease which may or may not be associated with apparent small vessel inflammatory or vasculopathic changes. The risk of secondary induced tumours runs at approximately 1 in 2000 cases. Numerous attempts to understand the critical factors have pointed clearly to dose and particularly fraction sizes as related to neurocognitive problems, with induced leucoencephalopathy, and damage to small blood vessels markedly increasing above fraction doses of >2 Gy [13, 14]. There thus becomes the issue of the burden of radiation therapy duration in palliative-intent patients versus the increased risk of side effects from shortening treatment regimens by using higher dose fractions. Usual current fractionation schedules thus compromise to deliver 30 Gy in 10 fractions where the aim is rapid stabilisation for palliative control and 40 Gy in 20 fractions where the aim is longer-term disease control.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







