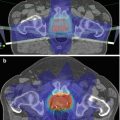
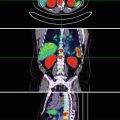
Fig. 5.1
A normal 18F-choline PET scan (MIP image). Physiological uptake of choline is present in the salivary glands, liver, spleen, kidneys, bowel and bladder with low-grade normal bone marrow activity
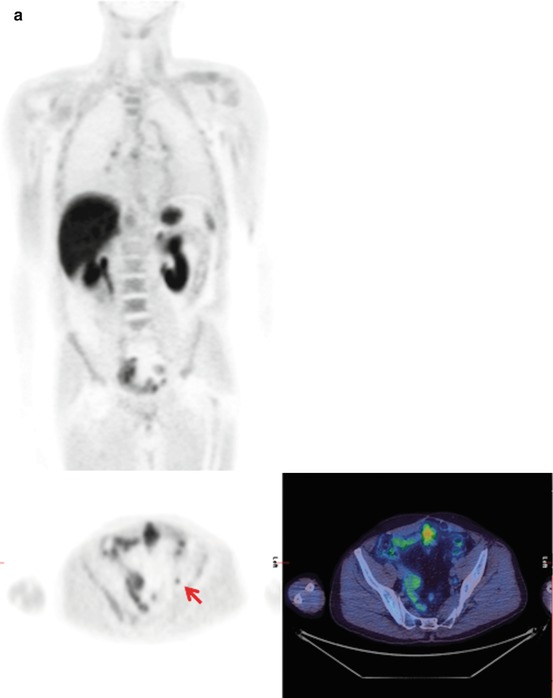
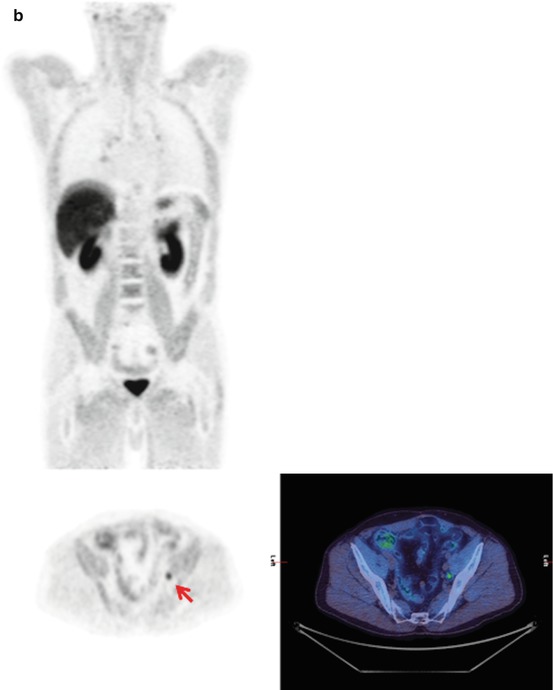
Fig. 5.2
(a) 11C-choline (i) coronal PET and (ii) transaxial PET and fused PET/CT in the pelvis, (b) 18F-choline (i) coronal PET and (ii) transaxial PET and fused PET/CT in the pelvis. 11C-choline and 18F-choline PET/CT studies performed at an interval of 5 months in a 65-year-old male with rising PSA after previous prostatectomy for prostate cancer. Both tracers show a metabolically active 8 mm left external iliac lymph node (arrow), appearing more prominent on the later 18F-choline scan, in keeping with nodal disease recurrence. In addition, in both studies there is low-grade mediastinal/hilar and inguinal nodal uptake in keeping with nonspecific reactive changes. Note slight differences in biodistribution between the two tracers. There is less urinary excretion and muscle activity with 11C-choline
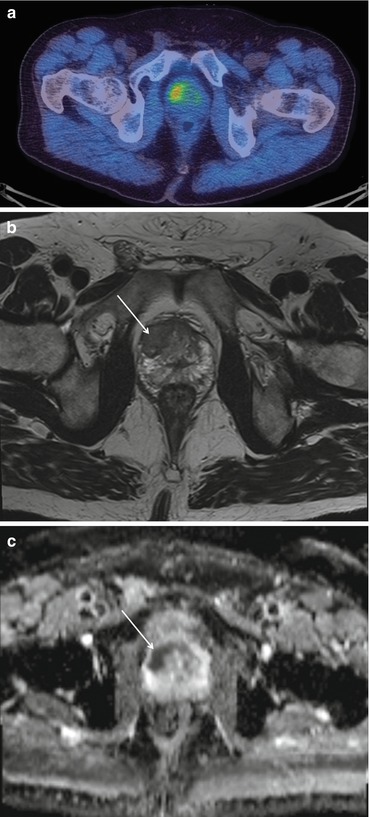
Fig. 5.3
A patient with a new diagnosis of prostate cancer. The 18F-choline scan shows abnormal uptake in the primary cancer in the right side of the prostate gland (a). This corresponds with an area of low signal of the T2-weighted (b) and restricted diffusion on the ADC (c) MRI scans
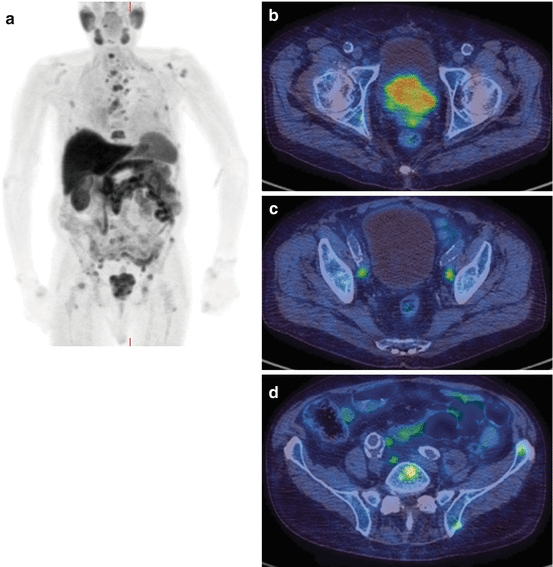
Fig. 5.4
A patient who presented with prostate cancer and a PSA of 300 ng/ml. The 18F-choline PET/CT shows abnormal uptake in the prostate gland, lymph nodes and skeletal metastases. MIP (a), pelvic axial fused images (b), prostate (c), pelvic nodes (d), iliac nodes and bone metastases
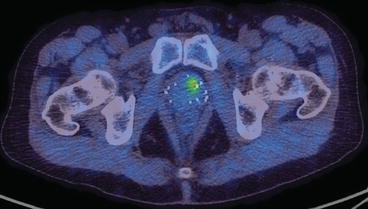
Fig. 5.5
A patient previously treated with brachytherapy subsequently was found to have a rising PSA. The 18F-choline scan showed recurrent disease within the prostate gland but no areas of nodal or distant metastatic disease

Fig. 5.6
18F-choline axial CT, PET and fused PET/CT images of a 72-year-old man previously treated with brachytherapy for prostate cancer with a subsequent rising PSA. The images demonstrate focal activity in the seminal vesicle on the left (arrow) indicating recurrent disease
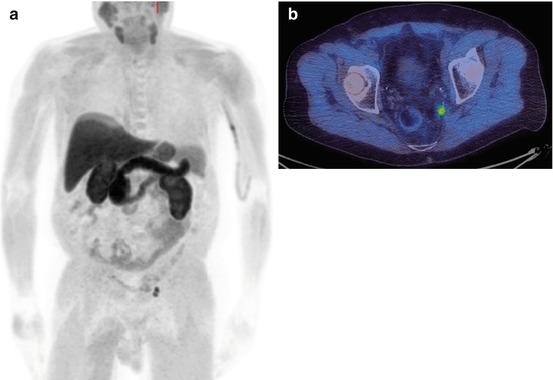
Fig. 5.7
A patient with a rising PSA 1 year after a radical prostatectomy. The 18F-choline PET/CT scan shows small volume recurrent nodal disease in the left side of the pelvis on the MIP image (a) and axial fused image (b)
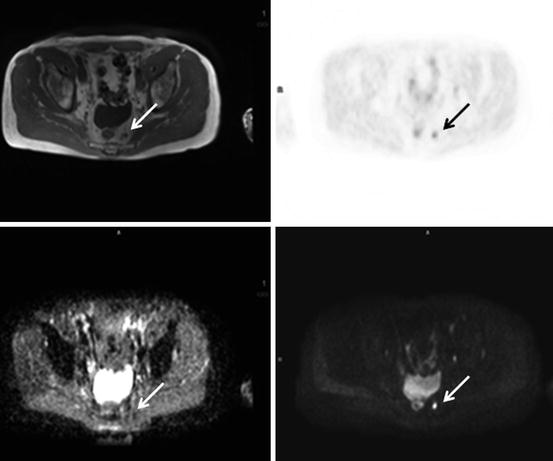
Fig. 5.8
18F-choline PET/MRI scan with axial pelvic images, T2 (top left), PET (top right), ADC map (bottom left), b900 image (bottom right). A 68-year-old man with previous prostatectomy for prostate cancer and subsequent rising PSA. The images demonstrate a left presacral nodal recurrence (arrows) with high 18F-choline activity, low ADC and high signal on the b900 diffusion-weighted image
Acetate is a substrate for numerous cellular processes, including the anabolic pathway leading to fatty acid synthesis. Radiolabelled acetate tracers have demonstrated utility in imaging prostate cancer, including in patients with lower PSA levels, but such tracers are neither cancer nor prostate specific.
There is increasing interest in more prostate-specific tracers, including prostate-specific membrane antigen (PSMA)-targeted imaging tracers, and those targeting androgen receptors (Figs. 5.9, 5.10, and 5.11). PSMA are type II transmembrane proteins, overexpressed in prostate cancer [4].
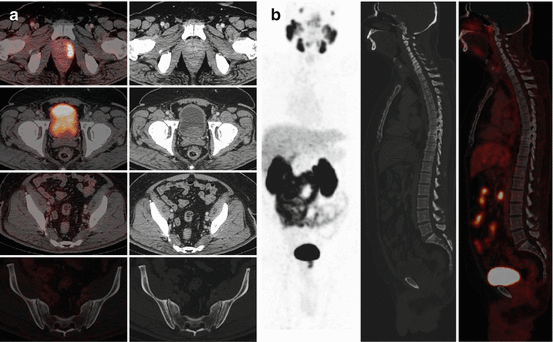

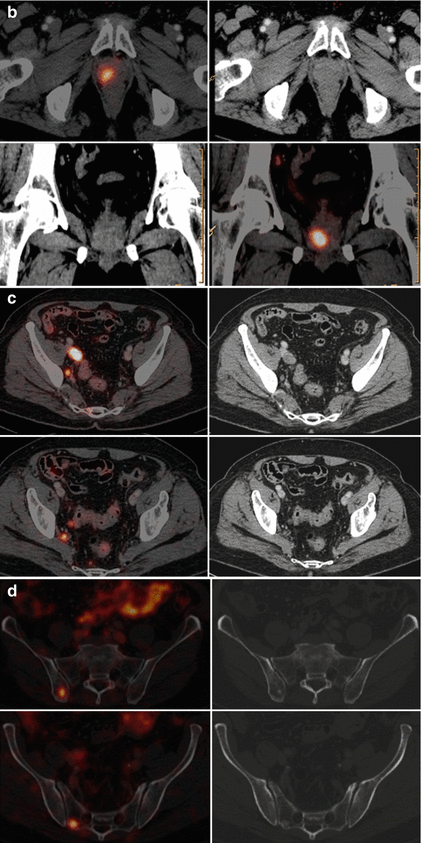
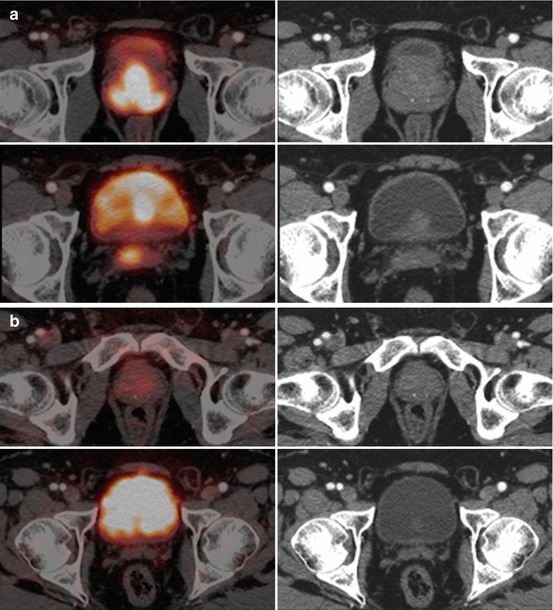

Fig. 5.9
68Ga-PSMA PET/CT. (a) Axial fused PET/CT and CT images through the pelvis and (b) PET MIP and sagittal CT and fused PET/CT images. A man with recently diagnosed adenocarcinoma prostate adenocarcinoma (Gleason score: 4 + 4). There is an intensely 68Ga-PSMA avid lesion involving the left anterior and posterior peripheral zones of the prostate gland. (b) There are no other 68Ga-PSMA avid lesions in the rest of the body. Physiological 68Ga-PSMA distribution with physiological uptake in the lacrimal, salivary glands, liver, bowel loops, kidneys and urinary bladder


Fig. 5.10
68Ga-PSMA PET/CT. (a) PET MIP, (b) axial (top) and coronal (bottom) fused PET/CT and CT through the prostate gland, (c) axial PET/CT and CT images through the pelvis and (d) axial PET/CT and CT images (bone windows) through the pelvis. A man with a diagnosis of Gleason 5 + 4 prostate cancer. The images demonstrate a primary prostate cancer in the right peripheral zone (a, b), right external and internal iliac nodes (c) and right iliac and sacral bone metastases (d)

Fig. 5.11
68Ga-PSMA PET/CT. (a) axial PET/CT and CT through the prostate gland and seminal vesicles and (b) same images after treatment with hormone therapy. The baseline images (a) show primary cancer in the peripheral and central zones as well as the right seminal vesicle. After hormone therapy (b) there is a marked reduction in activity at all sites of disease
Other tracers have shown potential utility for prostate cancer imaging, including markers of amino acid transport (e.g. the leucine analogue, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, 18F-FACBC), cellular proliferation (e.g. 18F-fluorothymidine (FLT)), hypoxia (18F-fluoromisonidazole) and angiogenesis (RGD-based tracers). Such tracers have a potential role in prostate cancer management.
5.1 Diagnosis of Prostate Malignancies
The current diagnostic tools of serum prostate-specific antigen (PSA), digital rectal examination (DRE) and transrectal ultrasound scan (TRUS)-guided biopsies to provide pre-surgical tumor grading of prostate cancer are only accurate in around 69% of patients [5].
Most prostate malignancies show increased uptake of choline-PET tracers. However, uptake in benign prostate hypertrophy has been shown, and some report an inability of these tracers to differentiate between benign and malignant prostate tissue [6]. A sensitivity of up to 90% and specificity of 86% have been reported for the detection of localized prostate malignancy [7] with choline PET/CT, but the accuracy is lower for smaller tumors.
There is not enough evidence currently to support the use of choline-PET/CT, or other tracers, for screening patients for malignancy. There may be a role for guiding biopsies in patients who have repeated negative prostate biopsies despite a high clinical suspicion [8] but this remains an area of research interest at present.
5.2 Staging of Prostate Malignancies
Given the uncertainty of the accuracy of choline-PET in differentiating benign from malignant tissue, the value of this technique for T-staging prostate tumors is limited. The spatial resolution of clinical PET/CT scanners in widespread use is insufficient to accurately assess the prostate capsule for evidence of involvement or breach. The development of PET/MRI may show T-staging benefits, as suggested in a 15-patient feasibility study using 18F-choline PET/MRI [9] (Figs. 5.3 and 5.8).
Identifying involved lymph nodes at diagnosis (N-staging) has significant clinical significance, but has been difficult to achieve accurately with all imaging modalities, including MRI. Contractor et al. showed that 11C-choline PET/CT was more sensitive than MRI for nodal staging (p = 0.007), detecting more sub-centimeter involved nodes [10]. Whilst choline-PET/CT has demonstrated good specificity, the sensitivity is relatively low and is dependent on the size of the involved lymph node and the PSA levels. De Jong et al. reported sensitivity/specificity values of 80%/96%, respectively, but the mean PSA for the 67 patients studied was over 100 ng/ml. In contrast, Beheshti et al. reviewed 130 patients with a mean PSA of 27 ng/ml (suggesting earlier disease and/or less disease burden) and reported a sensitivity of only 45% for nodal analysis, but a specificity of 96% [8]; the sensitivity increased to 66% if only nodes larger than 5 mm were considered. Other studies have demonstrated similar values of sensitivities and specificities [10–12].
The diagnosis of distant metastatic disease has important treatment implications; metastatic prostate cancer is incurable, and therefore invasive and morbid treatment to the primary disease is unlikely to be appropriate. Prostate cancer most frequently spreads to the bone causing typically sclerotic deposits. The current most common imaging method for screening for metastatic bone disease is with standard scintigraphy using technetium-labeled diphosphonates which are incorporated into the bone matrix of metastatic deposits secondary to the excess osteoblastic activity. 18F-fluoride, as a PET tracer, has a similar mechanism of uptake, but offers potential benefits from the resolution of PET/CT imaging, offering quantification potential and providing tomographic information as routine (Fig. 5.12). Faster clearance also allows imaging as early as 1 h post-injection. Choline PET/CT has been compared with standard bone scintigraphy in prostate cancer patients; Picchio et al. reported a sensitivity for identifying bone metastases of 89% for 11C-choline PET/CT and 100% for bone scintigraphy, but the specificity was much greater for 11C-choline PET/CT at 98 vs. 75% for bone scintigraphy [13]. Similar results have been reported by other groups [14]. This advantage of choline as a tracer is likely because there is little increased uptake in chronic degenerative lesions, unlike with standard 99mTc bone scintigraphy. Beheshti et al. reported that in one study, 18F-choline PET/CT identified early bone marrow involvement that was not visible on CT alone [8]. No evidence currently demonstrates the superiority of choline PET/CT compared with standard staging techniques for the identification of bone metastases from prostate cancer, but it may have value in certain individual cases for problem solving (Fig. 5.4).
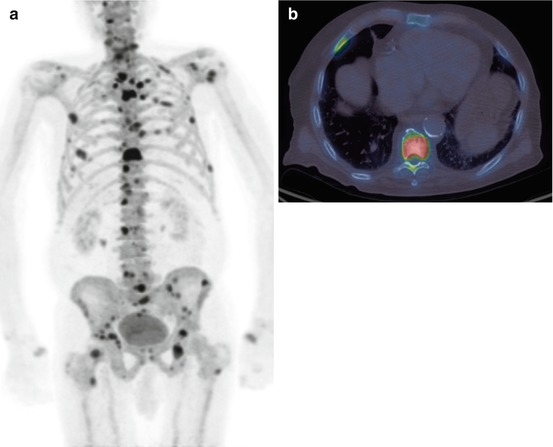

Fig. 5.12
A 18F-fluoride PET/CT scan (a) MIP image and (b) axial fused image at the level of the lower thoracic spine showing multiple bone metastases. This is the same patient as in Fig. 5.4
5.3 Restaging at Disease Recurrence
Imaging needs to identify sites of disease relapse, in particular whether this relapse is local to the prostate, within local or distant lymph nodes or distant metastatic spread. This has important treatment implications; a confined local recurrence might still be cured with salvage treatment. It is not uncommon for prostate cancer patients to have disease recurrence suspected by serial serum PSA rises. TRUS-guided biopsy only detects local recurrence in about 25–54% of these patients and is particularly poor when PSA values are low [15, 16]. CT has only a low diagnostic accuracy for localizing recurrent disease [17].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree