× 100
The gland weight in grams can be estimated by palpation, by ultrasound, or by nuclear imaging; in practice the first two methods are most commonly used. The desired dose to the thyroid tissue is expressed as the activity desired per gram of tissue (μCi/g or MBq/g), and it has ranged in studies between 90 and 200 μCi/g or 3.33–7.4 MBq/g. An activity of radioiodine between 150 and 200 μCi is usually recommended [11]. Several studies have reported outcomes of RAI given as fixed doses or calculated doses in hyperthyroidism [16, 17]; however, head-to-head trials comparing both methods are scarce. A study comparing different protocols of RAI therapy in toxic adenoma found a higher cure rate in the calculated high-dose protocol compared to a calculated low-dose, fixed high-dose, or fixed low-dose protocol [18]. At the same time, the rate of hypothyroidism was higher in the calculated and fixed high-dose groups compared to the lower-dose groups. A systematic review and meta-analysis of studies comparing fixed doses or estimated doses, based on thyroid size, to calculated doses in patients with hyperthyroidism showed no significant differences in cure of hyperthyroidism between the two methods. They included in the analysis patients with Graves’ disease, TMNG, and AFTN [19].
Pretreatment with Antithyroid Drugs
The antithyroid drugs (ATDs), methimazole, propylthiouracil, and carbimazole, inhibit the synthesis of new thyroid hormone from the thyroid gland, thus controlling the hyperthyroidism of TMNG, AFTN, or Graves’ disease. In the case of TMNG and toxic adenoma, they are frequently used before definite therapy with RAI.
In theory, RAI therapy can exacerbate signs and symptoms of thyrotoxicosis; therefore, the use of ATDs depletes thyroid hormone stores and can potentially ameliorate or prevent exacerbation of hyperthyroidism induced by RAI therapy. The benefit of this approach has not been thoroughly investigated in RCTs, and the use of ATDs in this setting is controversial with some advocating against the use of ATDs given the lack of evidence of benefit. Still some institutions use them routinely before administration of RAI. It is thought that RAI therapy exacerbates symptoms of thyrotoxicosis through induction of radiation thyroiditis. In a study of 34 patients with hyperthyroidism (11 with TMNG, 2 with AFTN, and 21 with Graves’ disease) who didn’t receive ATDs, all patients with TMNG had elevations of thyroid hormones after RAI compared to only 29 % of those with Graves’ disease. However, none had worsening symptoms of thyrotoxicosis [20]. Despite these findings there are reports in the literature of thyroid storm and severe hyperthyroidism after RAI [21, 22].
When considering pretreatment with ATDs, three main factors need to be addressed: (1) the severity of hyperthyroidism; (2) the patient’s comorbidities such as cardiovascular disease, cardiac arrhythmias, patient’s age, and overall health; and (3) the risk of decreasing the effectiveness of RAI in patients taking ATDs.
Patients with severe hyperthyroidism, elderly patients, or patients with cardiac disease are at higher risk of developing complications from exacerbation of the hyperthyroid state. Consequently these are the patients in which pretreatment with ATDs should be considered.
If ATDs are continued at the time of RAI, there is clear evidence that the cure rate in TMNG and AFTN is significantly lower compared to when no ATDs are given [23]. However, whether ATDs reduce the efficacy of 131I if they are stopped a few days before therapy is controversial. A systematic review and meta-analysis of 14 randomized controlled trials of the effect of ATDs on RAI found that the adjunctive use of ATDs was associated with higher RAI failure rates compared to no ATDs [24]. However, only 5/14 studies included patients with TMNG or AFTN, and 5 of the included studies gave ATDs concomitantly with RAI.
The most common practice is to discontinue ATDs before RAI, and the time interval between discontinuation of ATD and RAI therapy varies among studies between 2 and 7 days. If carbimazole is stopped 3 days before RAI, the cure rates were similar than if no ATDs were given [25].
If ATDs are given before RAI, it is preferred to use either methimazole or carbimazole instead of propylthiouracil (PTU) given the higher risk of liver dysfunction [26] and evidence from observational studies, suggesting radioresistance of the thyroid gland with PTU [27].
ATDs can be given for several weeks until hyperthyroidism is controlled; however, TSH should not be normalized to avoid the inadvertent therapy of the normal thyroid tissue, thus increasing the long-term risk of hypothyroidism. ATDs should be discontinued 3–7 days before RAI [11] and reinitiated 7 days later if there are concerns about risks associated with transient increase in thyroid hormone values. The dose of ATDs should be gradually decreased with the goal to discontinue them 1 month after RAI.
Should rhTSH Be Used to Augment RAI Uptake and Efficacy?
Recombinant human TSH (rhTSH) is commonly used with RAI ablative therapy after thyroidectomy for thyroid cancer. In the case of TMNG, the use of rhTSH has been considered in cases of low RAI uptake to increase the uptake and potentially increase the efficacy of RAI and the thyroid absorbed dose. Observational studies [28, 29] and one RCT [30] using rhTSH before RAI have included patients with nontoxic multinodular goiter and a subset of patients with TMNG with subclinical hyperthyroidism or mild hyperthyroidism. The RAI uptake increased two- to fourfold 24 h and 72 h after rhTSH, respectively. No clinical worsening of hyperthyroidism was reported after 0.1 or 0.3 mg of rhTSH. However, thyroid hormone levels increase after rhTSH, and exacerbation of hyperthyroidism can occur [31]. Consequently, more studies evaluating the safety and efficacy of rhTSH augmented RAI in TMNG compared to RAI alone are needed before recommending its use.
Risk of Hypothyroidism
The risk of hypothyroidism persists years after treatment with 131I. Rates of subclinical hypothyroidism or overt hypothyroidism as high as 72 % have been reported 8 years after RAI [32]. Higher doses of RAI are more likely to cause hypothyroidism [18, 32]. For TMNG the rate of hypothyroidism 5 years after treatment is between 7 % and 14 % for lower and higher doses of RAI, respectively [7, 33, 34]. The average rate of hypothyroidism is 2.7 %/year with 64 % of patients hypothyroid 24 years after RAI [35].
For autonomously functioning thyroid nodules, the rate of hypothyroidism is between 7 % and 55 % when lower and higher doses of RAI are given, respectively [17, 36], and the cumulative incidence of hypothyroidism is 7 % at 1 year, 28 % at 5 years, and up to 60 % at 20 years [37]. Besides the dose of RAI administered, other risk factors associated with higher rates of hypothyroidism include the presence of thyroid antibodies, a smaller or non-palpable thyroid gland, and the use of ATDs before 131I [6, 34, 38].
To prevent hypothyroidism, ATDs combined with thyroid hormone were used in 149 patients, with a reported rate of overt hypothyroidism of 3.3 %, 6.8 years after RAI [39]. The idea of this approach was to first control hyperthyroidism with ATDs and then give enough thyroid hormone to suppress TSH to avoid RAI uptake by normal thyroid tissue. This approach has not been validated and is therefore not recommended. Because the risk of hypothyroidism after RAI in TMNG and AFTN is significant, thyroid function tests should be periodically monitored even after clinical and biochemical evidence of euthyroidism.
Contraindications to RAI
Absolute contraindications to RAI therapy for hyperthyroidism from TMNG or toxic adenomas are pregnancy, concomitant diagnosis of thyroid cancer (for which surgery is the most appropriate therapy), women planning pregnancy within the next few months of therapy with RAI, or people unable to comply with radiation safety procedures [11].
Risks of RAI
Cancer Risk
The Cooperative Thyrotoxicosis Therapy Follow-Up Study has followed patients treated for hyperthyroidism in 26 medical centers in the United States and England. At 6 years of follow-up, the incidence of thyroid cancer mortality and leukemia was not increased in the patients treated with 131I compared to the patients treated with other modalities [40, 41]. At a mean follow-up of 21 years, there was no difference in overall cancer mortality in the 131I-treated patients compared to the general population. However, there was a small but significant increase in thyroid cancer deaths (SMR 3.94. CI: 2.52–5.86) in the 131I-treated patients [42]. Patients who died from thyroid cancer were more likely to have had toxic nodular goiter, suggesting that the increased risk in thyroid cancer might be related to nodular goiter itself and not to RAI.
Mortality and CVD Mortality Risk
Population-based studies have found an increased risk in CVD and all-cause mortality in patients with hyperthyroidism treated with 131I when compared to mortality estimates in the general population [43, 44]. A major limitation in these studies is the inability to differentiate the contribution of the RAI treatment itself to the increased mortality which could be solely explained by hyperthyroidism.
Induction of Thyroid Autoimmunity
Appearance of Graves-like disease after RAI for TMNG or AFTN has been repeatedly reported. Its occurrence is rare between 1 and 4 % [45, 46]. It usually manifests 3–6 months after RAI with elevation of thyroid hormone levels, appearance of thyrotropin receptor antibodies (TRAB), and a thyroid scan showing diffuse radioiodine uptake.
Thyroglobulin, a protein synthesized solely by follicular cells in the thyroid gland, is released to the systemic circulation after RAI. Therefore, it is thought that an increase in thyroid antigens following RAI may stimulate an immune response toward the TSH receptor.
In a study evaluating the risk to develop Graves’ disease after RAI, the phenotype occurred more commonly for TMNG than for toxic adenoma with rates of 2 % and 0.3 %, respectively, but none of those with toxic nodular goiter that didn’t receive 131I developed the disease [46]. This finding suggests that RAI therapy induces the thyroid autoimmune response. If a patient with TMNG or toxic adenoma develops recurrence of hyperthyroidism months after RAI therapy, it is much more likely to be from recurrence of autonomy than Graves’ disease. However, we recommend getting a thyroid 121I uptake and scan, and if there is diffuse uptake throughout the gland, Graves’ disease is more likely, and treatment with ATDs or retreatment with RAI should be considered.
Marine-Lenhart Syndrome
The diagnosis of this entity is usually made by the presence of hyperthyroidism, positive thyrotropin receptor antibodies, and thyroid nodules seen on ultrasound and thyroid uptake and scan. The treatment for Marine-Lenhart syndrome follows the same principles discussed. Radioactive iodine and surgery are the most commonly used therapies. The dose of RAI required for cure is usually similar to the dose required for TMNG or AFTN, which is higher than the dose required for Graves’.
Role of Antithyroid Drugs
Mechanism of Action
The thionamide compounds available in the United States are methimazole (MMI) and propylthiouracil (PTU). Carbimazole (CBZ) is another thionamide available and commonly used in some European and Asian countries. These compounds alleviate hyperthyroidism by inhibiting thyroid hormone synthesis. They inhibit the oxidation from iodide to iodine and iodine organification catalyzed by thyroperoxidase, a necessary step for the incorporation of iodine into thyroglobulin. Thionamides also inhibit coupling of iodotyrosines and alter the structure of thyroglobulin [47]. Carbimazole is metabolized to methimazole in serum. Propylthiouracil, but not methimazole or carbimazole, inhibits the conversion from T4 to T3 in extrathyroidal tissues.
Efficacy
ATDs control hyperthyroidism but don’t cause remission in toxic nodular goiter. After 12 months of therapy with ATDs, 95 % of patients with TMNG have recurrence of hyperthyroidism when ATDs are discontinued [48]. If ATDs are chosen as the main treatment, lifelong therapy will be required. Accordingly, surgery, radioactive iodine, or other ablative therapies are usually preferred over ATDs in the treatment of TMNG and AFTN (see Table 8.1). A study evaluating the effects of long-term therapy with methimazole in diffuse toxic goiter compared to RAI showed that methimazole therapy for 10 years is overall safe. Euthyroidism was achieved in 93 % of patients on MMI [49]. There were more thyroid test abnormalities at follow-up in the group that received RAI. However, there was a high number loss to follow up over all.
Table 8.1
Preferred treatment option for toxic multinodular goiter (TMNG) or autonomously functioning thyroid nodule (AFTN) according to specific clinical factors
Therapy options | ||||
|---|---|---|---|---|
Clinical factors | Surgery | Radioactive iodine (RAI) | Ablative therapiesa | Antithyroid drugs |
Contemplating pregnancy within few months | ✓✓✓ | –b | ✓OK to consider for AFTN | – |
Goiter with compressive symptoms | ✓✓✓ | ✓✓ | ✓OK to consider for AFTN | – |
Short life expectancy | – | ✓ | ✓✓✓ | ✓✓✓ |
Multiple comorbidities | ✓ | ✓✓✓ | ✓✓✓ | ✓ |
Previous neck/thyroid surgery | ✓ | ✓✓✓ | ✓ | ✓ |
Severe hyperthyroidismc | ✓✓✓ | ✓✓✓ | – | ✓ |
Nevertheless, long-term therapy with ATDs can be considered in elderly individuals with cardiac disease or other significant comorbidities when the risk for radioactive iodine or surgery is high. This is of particular importance for TMNG, because other therapeutic modalities to be discussed later, such as radiofrequency or thermal ablation, are effective in toxic adenomas but less so in TMNG.
Side Effects
Allergic reactions such as pruritus, rash, urticaria, and arthralgias occur in 5 % of people taking these medications and are the most common side effects. Agranulocytosis, polyarthritis, vasculitis, and hepatitis are rare but serious side effects. Agranulocytosis, which is an idiosyncratic reaction, occurs in 0.2–0.5 % with MMI, CBZ, or PTU [50]. Polyarthritis, vasculitis, and fulminant hepatitis are more commonly reported with PTU than MMI (frequency <1 %) [50]. Fulminant hepatitis occurs in 1:10,000 adults on PTU and is more common in children [26]. Liver dysfunction can also occur with MMI and is usually a cholestatic pattern [51]. Given the concern of significant hepatitis with PTU associated with deaths and liver transplantation, in most circumstances, MMI and CBZ are the first-line drug therapies for hyperthyroidism.
Role of Ablative Therapies
A large number of patients with nodular thyroid disease are advanced in age and have non-thyroidal comorbidities that increase the risk of a surgical intervention. It is also not uncommon for these patients to have large nodules without a very high uptake of radioactive iodine which leads to the need to use large doses of RAI (by comparison with Graves’ disease patients). On this background the developments of ultrasonography over the last couple decades has allowed the creation of procedures that target the thyroid nodules percutaneously with delivery of high energy or chemical agents in the nodular tissue under direct ultrasound visualization. The main area of use for these procedures has been the benign, nontoxic thyroid nodules. However, these procedures have also been employed in patients with toxic nodules that for cultural or other nonmedical issues have refused surgery or RAI. In almost all studies, the targeted nodules have been large nodules, many with documented compressive symptoms or at risk of producing such symptoms. The ultrasound-based procedures targeting thyroid nodules are radiofrequency ablation (RFA), percutaneous laser ablation (PLA), and percutaneous ethanol injection (PEI). Table 8.1 summarizes the clinical situations where the interventions described here could be considered.
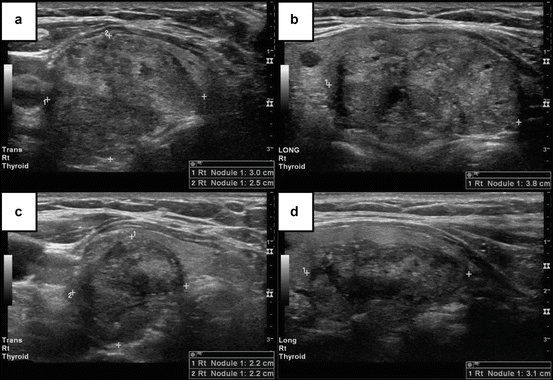
Radiofrequency Ablation
Radiofrequency ablation or radiofrequency thermal ablation (RFA) is the most utilized of the ablative therapies. Its use is based on the principle that ultrasound-based energy delivery into the thyroid tissue will raise the local temperature to about 101–105°C which will lead to local thrombosis followed by ischemia and fibrosis and subsequent shrinkage of the treated tissue volume (see Fig. 8.1). The needles employed are 14–18 G, some allowing further deployment of one to four expandable hooks that are then making contact with multiple portions of the targeted nodule. The procedure in all cases is performed under continuous ultrasound guidance. There are at least two techniques in practice: fixed-needle technique and “moving-shot” technique which are distinguished by the degree of manipulating the needle during the procedure. The energy delivered per nodule is a variable between reports, and it has been delivered for most patients in multiple sessions (mean is about two sessions/nodule from combining multiple studies data). The sessions were repeated based on thyroid function and/or nodule size response and performed 1–2 months apart. Local anesthesia with 2 % lidocaine has been employed for the superficial cervical tissue and the thyroid capsule. In most cases the cystic content, if present, is aspirated before the energy is delivered. No significant side effects or hospitalization was noted in these patients. A sensation of heat in the neck was reported by patients. That did not affect the course of the procedure. If pain developed the power was decreased or ablation stopped completely for few seconds. Some reports described repeat biopsies prior to procedure to ensure the benign character of the treated lesions.