8.1
Introduction
The skeleton is essentially responsible for providing not only structural support and protection to the body’s organs but also for serving as a reservoir for calcium, magnesium, and phosphate; ions that are of critical importance in physiology. The fabric of bone is a unique composite of living cells embedded in a remarkable three-dimensional (3D) structure of extracellular matrix, stabilized by mineral that is a carbonate-rich analog of the geologic mineral, hydroxyapatite (HA). Because of the vast expansion of literature in this field, this chapter relies primarily on reviews. Readers are referred to the earlier edition of this book for most primary references.
8.1.1
Bone tissue: composition
During development, embryonic mesenchymal cells form the skeleton via two basic pathways . Intramembranous bone is formed by direct differentiation of mesenchymal cells into bone, whereas endochondral bone is formed by a condensation of mesenchymal cells that leads to the creation of a cartilaginous structure. Serving as a temporary model, the cartilage becomes calcified (hypertrophic), and the provisional calcified cartilaginous precursor is subsequently replaced by bone. A third less recognized pathway acting primarily in the cranium, but also at other sites in the skeleton, involves regression of unmineralized cartilage by apoptosis, and subsequent remodeling of remaining cells into other tissues, including bone .
Invasion by blood vessels brings in the cells that remove bone (osteoclasts) and, in addition, the osteoblastic precursors that will replace the calcified cartilage with bona fide bone. The initial bone formed, woven bone, is a rather unorganized conglomeration of collagenous and noncollagenous proteins that induce the precipitation of mineral. Through modeling by osteoclasts, this primordial bone is removed and replaced by lamellar bone, a more highly organized structure with alternating layers of mineralized extracellular matrix, whose plywood-like structure provides bone with its mechanical strength.
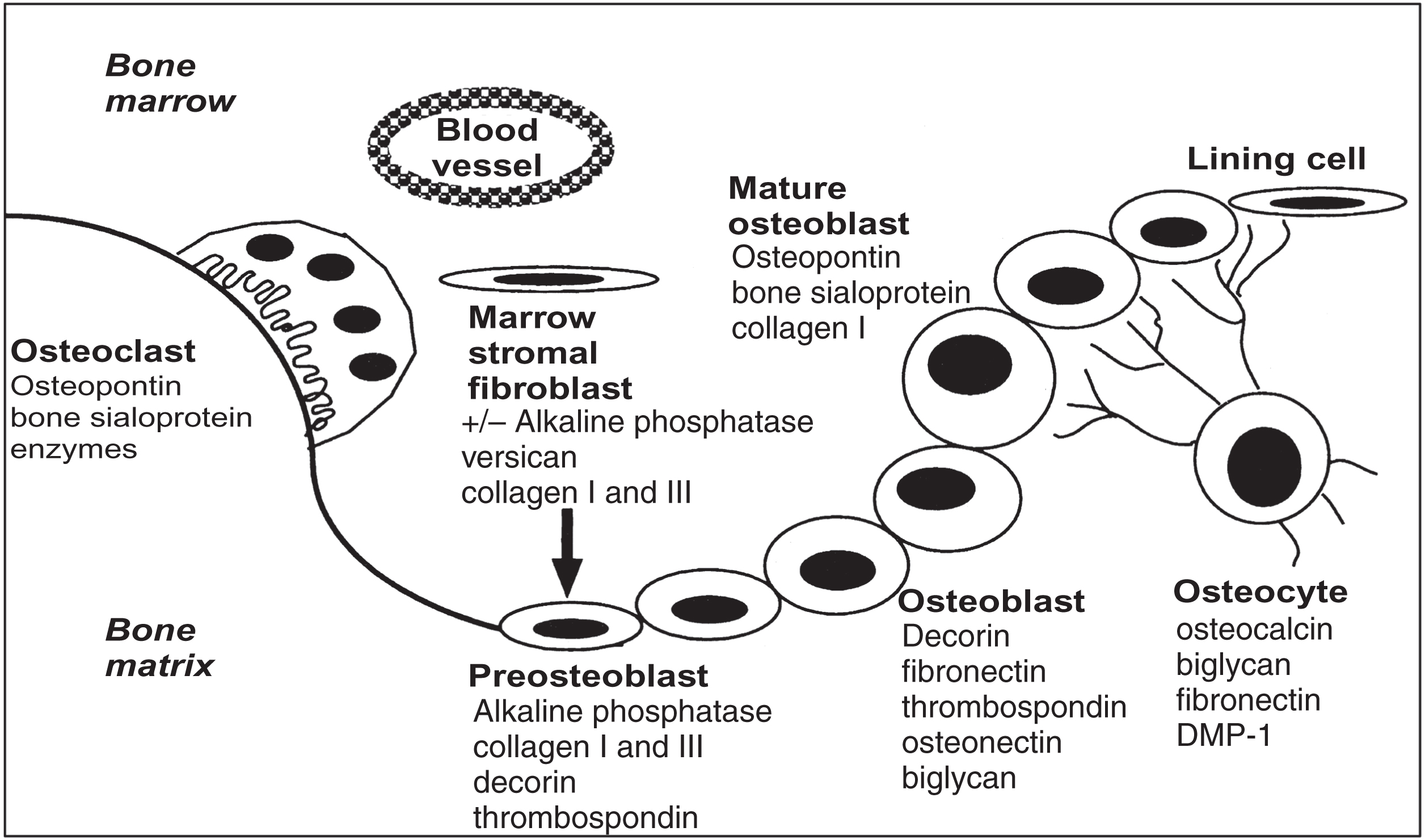
Extracellular matrices are diverse structures underlying and/or surrounding cells and are composed of diverse proteins that often have repeating domains that are shared by different proteins . Although the mineralized extracellular matrices (calcified cartilage, bone, dentin, cementum, and enamel) were originally thought to be composed of unique sets of matrix proteins, the majority of these proteins are synthesized by nonskeletal cells as well. Bone is composed of 70%–90% mineral and only 10%–30% protein, with collagenous protein comprising ~90% of the bone matrix and noncollagenous proteins accounting for the remaining ~10%. However, many of the collagenous and noncollagenous proteins in bone differ from those in other tissues in their chemical nature. These diverse forms are a result of alternative splicing of mRNA and different posttranslational modifications, such as glycosylation [including the covalent attachment of glycosaminoglycans (GAG)], phosphorylation, and sulfation. These chemical differences most likely influence the physiological function of these proteins, and the appropriate mixture provides bone matrix with the ability to calcify. Moreover, because most of the extracellular matrix proteins (ECMs) are the secretory products of cells in the osteoblastic lineage, they represent biochemical markers of maturation stages of cells during the formation process ( Fig. 8.1 ) or the resorption process (in their degraded form) of bone. Comparisons of younger and less mature bone even show a difference in the nature of the noncollagenous proteins expressed in the same tissues . This chapter describes the major types of proteins in bone matrix synthesized by osteogenic cells and discusses their potential roles in the regulation of mineralization.
8.1.2
Bone mineral
Deposition of mineral crystals solidifies the organic matrix, giving bone its unique mechanical properties. The crystals are similar to the geologic HA (Ca 10 [PO 4 ] 6 [OH] 2 ) ( Fig. 8.2 ), but they are smaller, form very thin needles and plates, and contain numerous substitutions with ions not found in pure HA . This structural organization (often referred to as poor crystallinity) ensures higher solubility and faster ion exchange in bone compared with geologic HA, which is essential for normal homeostasis of Ca 2+ , Mg 2+ , <SPAN role=presentation tabIndex=0 id=MathJax-Element-1-Frame class=MathJax style="POSITION: relative" data-mathml='PO43−’>PO3−4PO43−
PO 4 3 −
, and other ions . It also enables bone formation and remodeling at biological rather than geological time scales. Amorphous calcium phosphate and octacalcium phosphate crystal deposits have been found in bone, but the substituted HA crystals are believed to be the main component of mature bone mineral . Mineral deposition and homeostasis in bone are tightly regulated by cells through numerous collagenous and noncollagenous matrix proteins, proteoglycans, and glycoproteins.
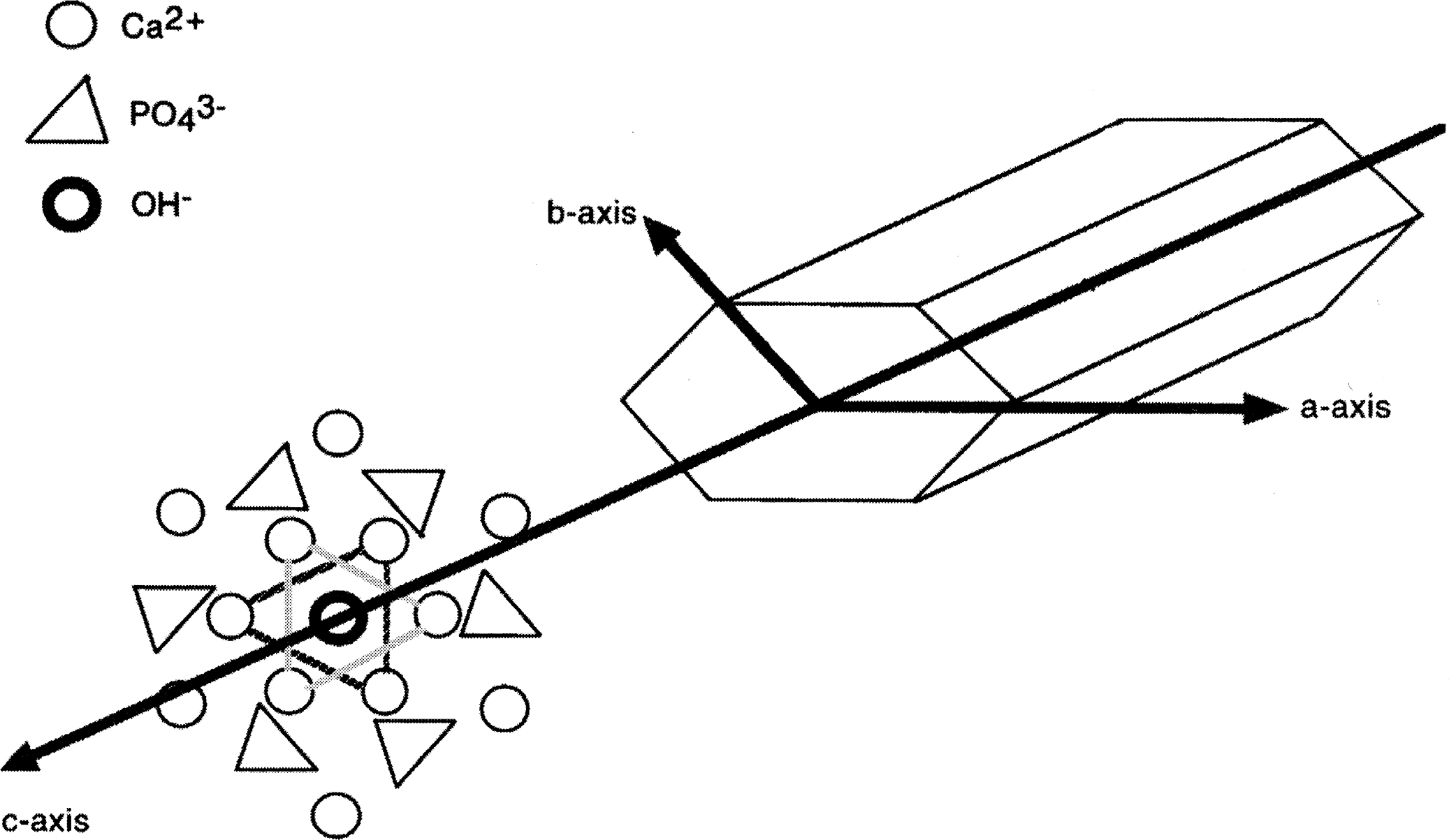
PO 4 3 −
ions about the OH − position.
8.2
Collagenous proteins
In the skeleton, the major structural protein is collagenous in nature. Collagen in bone is predominantly composed of type I collagen, which serves a mechanical function by providing tensile strength . Collagen may induce mineral deposition and serves as an important “backbone” in support of mineral deposition and the organization of crystal growth by providing appropriate scaffolding and orientation of nucleators of mineralization.
8.2.1
Structure of the collagen molecule
Collagen is defined as a trimeric molecule composed of α chain subunits. A significant feature of the component α chains is that their primary sequence contains a repeating triplet sequence, Gly-X–Y, where X is often proline and Y is often hydroxyproline. Collagenous proteins are either homotrimeric, composed of three identical α chains, or heterotrimeric, with two or three different α chains. Individual α chains coil together to form an extended rigid triple helical structure. The structure is stabilized by hydrogen bonding between OH groups on hydroxyproline residues and intrachain water, and by aldehyde-derived cross-links .
Currently, the collagen family is composed of 28 types (reviewed in Ref. ), of which types I, II, III, VI, X, XI, and XXVII are found in bone matrix proper, calcified cartilage and matrices associated with the vasculature . Based on their structural features, collagens can be generally divided into two groups: fibrillar and nonfibrillar. Fibrillar collagens are all synthesized as precursors that are proteolytically trimmed of noncollagenous ends to yield mature molecules. Collagen fibrils are formed via head-to-tail associations of the mature collagen molecules, and different types of fibrillar collagens share a strong structural similarity in that the major part of each molecule is formed by an uninterrupted triple-helical domain. Once fibers have been formed in the extracellular environment, they are further stabilized by the formation of inter- and intramolecular cross-links.
Fibrillar collagens (such as types I, II, III, V, and XI) are by far the most abundant forms and are formed in the interstitial spaces of connective tissues throughout the body . Type I collagen ([α1(I) 2 α2(I)]), the predominant collagen of skin, tendon, and bone forms the major scaffolding of virtually all connective tissues except cartilage; cartilage contains predominantly type II collagen ([α1(II) 3 ]) with limited amounts of other collagens. Type III collagen, composed of three identical α1(III) chains, is found in many tissues rich in type I collagen. Less abundant fibrillar collagens, types V and XI, associated with collagen types I and II, respectively, are located on the periphery of the collagen fibrils. Contrary to other fibrillar collagens, type VXI’s N-terminal extensions are retained and project onto the fibril surface. This feature, together with the appropriate molar ratios of types I/V and types II/XI collagens in fibrils, is important in the regulation of fibril diameter . Structural analysis of fibrillar type I collagen shows that individual collagen fibrils are aligned in a quarter-staggered array, with a 280 nm periodicity. As a result of the quarter stagger, there are gaps (holes) within the fibrillar structures, and it is in these gaps and in the overlapping regions adjacent to them (e band) that bone mineral crystals first appear . A recently discovered fibrillar collagen, type XXVII, seems to be involved in the transition from calcified cartilage into bone .
The nonfibrillar collagens are characterized by triple-helical domains that are either shorter or longer than those of the fibrillar types, and they contain stretches of nontriple-helical sequences (fibril associated collagens with interrupted triple helices) . One of these nonfibrillar collagens, type X collagen, is found in calcified cartilage. The localization of type X to hypertrophic chondrocytes is highly specific, but it does not appear to have a major role in cartilage calcification. Type IX is a minor constituent in cartilage. It is composed of three different types of α chains, α1(IX), α2(IX), and α3(IX), which form a short and a long triple helix joined by a flexible hinge region. A GAG chain is also attached to one of the α chains at the amino terminus, making this collagen a proteoglycan as well. Type IX has been found as a coating of type II collagen fibrils and covalently attached to it. Mice lacking type IX collagen show cartilage and bone abnormalities presumably associated with their altered fibril diameters . Type XII is similar to type IX but has three projections extending from the triple helix. In addition to associating with type II collagen, it may also be associated with type I fibrils in tendon. Type XIV is also structurally related to type IX collagen fibrils and also associates with type II collagen in cartilage.
8.2.2
Bone matrix collagen(s)
Bone matrix proper contains a rather limited array of collagen types ( Table 8.1 ). Bone matrix contains predominantly type I collagen, but other types are present at much lower levels compared to soft connective tissues. In all connective tissues the collagens serve mechanical functions, providing elasticity and strength for the component tissues . The importance of type I collagen in bone is well demonstrated by various forms of osteogenesis imperfecta (OI; brittle bone disease) in humans and in animal models, in which bone fragility has been associated with qualitative and quantitative alterations in the type I collagen genes, COL1A1, COL2A . In addition, some forms of OI are caused by mutations in genes that regulate posttranslational modifications of collagen, control formation of native collagen, its passage through the endoplasmic reticulum, and in the extracellular environment where cross-linking and mineralization take place (see Chapter 49 ). The mineral crystals in the bones of patients and transgenic animals with OI tend to be smaller than those in age-matched control bones . For example, in a naturally occurring mutant mouse (oim/oim) that has an [α1(I) 3 ] trimer as opposed to the normal [α1(I) 2 α2(I)] trimer, the pattern of initial mineral deposition and crystal growth along the collagen differs from normal. The crystals are smaller with more variable alignment than in normal mice . In addition, fibrils are generally thinner in OI patients and OI mice, which may be insufficient to provide nucleation and scaffolding sites for mineral deposition and can potentially translate into fragile bones. On the other hand, mutations of bone morphogenetic protein-1 (BMP1), an enzyme that cleaves the C-terminal propeptide, have excessive mineralization, perhaps due to an increase in space for mineral accumulation due to disorganized packing of the collagen molecules . Collagen has binding sites for the noncollagenous proteins that regulate mineralization, and these domains could be unavailable in mutant tissues . Since these noncollagenous matrix proteins that are “held” within the collagen matrix appear to initiate and regulate the mineral deposition in bone, the data from OI tissues clearly demonstrate the importance of collagen for providing a scaffold to organize the mineral.
| Collagen | Location/function | Molecular structure |
|---|---|---|
| Type I:[α1(1) 2 α(1)] and [α1(1) 3 ] | Constitutes 90% of the bone matrix | 67-nm banded fibrils |
| Acts as scaffolding and binds to other proteins that initiate hydroxyapatite deposition | ||
| Type III:[α1(III) 3 ] | Present only in trace amounts and can regulate collagen fiber thickness | 67-nm banded, coats type I fibrils |
| Type V:[α1(V) 2 α2(V)] and [α1(V)α2(V) α3V] | Their absence can result in collagen fibrils of larger diameter | 67-nm banded, coats type I fibrils in some tissues |
| Type X:[α1(X) 3 ] | Present in hypertrophic cartilage and can be involved in matrix organization via formation of the template for type I collagen | Probably fishnet-like lattice |
8.3
Intermediate cartilage matrix
Endochondral bone formation starts off with a cartilage template, and cartilage macromolecules can be in close proximity to forming bone and may actually be incorporated into the initial boney tissue . The basic scaffolding on which cartilage matrix is built is type II collagen. In addition, a number of proteoglycans have been identified in cartilage matrix.
Proteoglycans are a class of macromolecules characterized by the covalent attachment of long chains of repeating disaccharides that are often sulfated, termed glycosaminoglycans (GAGs) to a protein core. Based on the sugar composition of the repeating disaccharides, GAGs are divided into subtypes such as chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), and hyaluronan (which is unsulfated, and not bound to a protein core) ( Fig. 8.3 ).
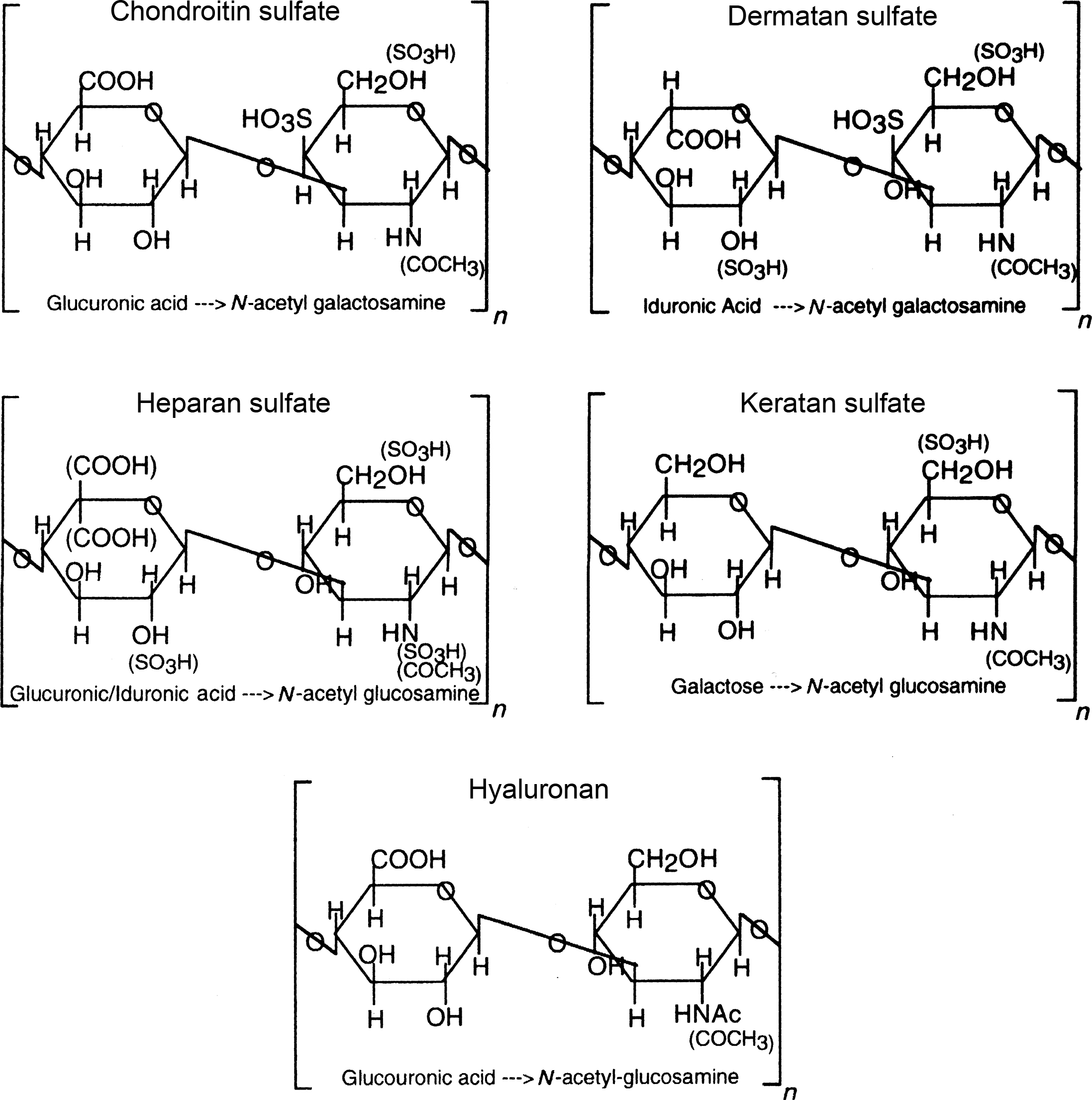
Most of the proteoglycans in cartilage are large proteoglycans belonging to the hyalectan/lectican class , such as ACAN (aggrecan), which is somewhat cartilage specific, and VCAN (versican), with smaller amounts of the small leucine-rich repeat proteoglycan (SLRP) proteoglycans, such as DCN (decorin), BGN (biglycan), ASPN (asporin), FMOD (fibromodulin), CHAD (chondroadherin), and PRELP (proline- and arginine-rich end leucine-rich repeat protein), all of which are also present in bone matrix. Other proteins, including COMP (cartilage oligomeric matrix protein), CD-RAP (cartilage-derived retinoic acid-sensitive protein), MATN1 and 3 (matrilin 1 and 3), FBLN (fibulin), and CILP-1 (cartilage intermediate layer protein-1), are present in cartilage matrix but at much lower levels than type II collagen and aggrecan .
8.3.1
Large proteoglycans
8.3.1.1
Aggrecan
Aggrecan is one of the large CS-containing molecules and has the ability to form aggregates with hyaluronic acid. Intact human aggrecan has a molecular weight of ~2.5 million Da, with a core protein ranging in apparent molecular weight between 180 and 370 kDa with slightly more than 100 GAG chains (mostly CS, but with some KS located near the N terminus) of ~25 kDa. Based on enzymatic cleavage and sequence homology, five domains have been defined in the core protein of aggrecan ( Fig. 8.4 ) . Two globular domains, G1 and G2, are near the N terminus and are separated by an interglobular domain that is sensitive to a number of proteases such as matrix metalloproteinases and cathepsin K . The G1 domain binds to hyaluronic acid and is structurally homologous to “link protein” , a small glycoprotein that stabilizes the interaction between the proteoglycan and hyaluronic acid in cartilage, forming a unique gel-like moiety providing resistance to compression in joints . Although the G2 domain is unique to aggrecan, its function is unknown. The G2 domain is followed by a stretch containing prolyl-serinyl repeats for attachment of KS-GAGs, and two stretches rich in serinyl–glycyl repeats to which the CS-GAGs are attached. The third globular domain is located at the C terminus and appears to be involved with aggrecan trafficking within and outside of the cell (reviewed in Ref. ).
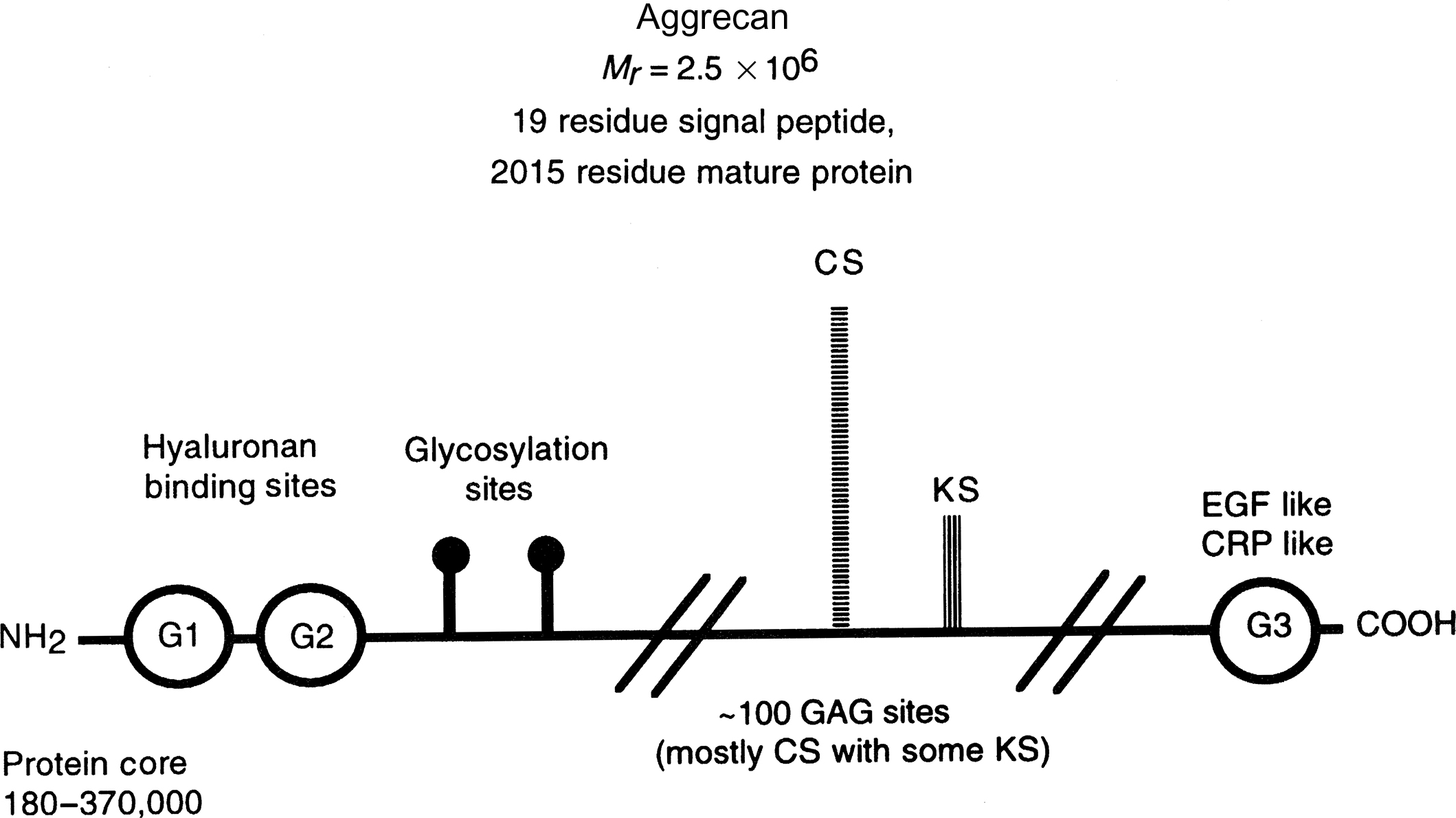
The importance of the structure of aggrecan is demonstrated by mutations in mice. Mice with cartilage matrix deficiency, which is caused by a functional null mutation of the aggrecan gene, are characterized by perinatal lethal dwarfism and craniofacial abnormalities, suggesting an important role for this proteoglycan in skeletal development . Mice lacking the ability to make hyaluronic acid in chondrocytes still form aggrecan, but they are unable to form a proper growth plate, cannot survive birth, and have major in utero skeletal deformities . Though less severe, mice that lack the ability to add CS to their core protein also have reduced growth plate widths and other skeletal abnormalities . In humans, ACAN mutations typically result in short stature due to growth plate cartilage abnormalities and can also lead to joint disease due to functional changes in articular and intervertebral disc cartilage (reviewed in Ref. ).
In addition to playing a hydrodynamic function, aiding in the retention of both water and cations and the exclusion of anions in cartilage, proteoglycans are also responsible for matrix maintenance and organization , in part through interactions with the GAG chains of type IX collagen that surround the type II collagen fibrils. Proteoglycans may also play a role in the regulation of cartilage calcification . The large aggregating cartilage proteoglycans can inhibit HA formation and growth in solution (e.g., ), and they can also chelate calcium and serve as a source of calcium ions for mineralization if they are degraded into non-Ca 2+ -binding fragments. Although there is debate as to whether this chelation is involved in the inhibition of mineralization, it is clear that proteoglycans and their component GAGs sterically block HA formation and growth .
The amount of aggrecan in bone is much lower than that in cartilage, and whether its presence in bone represents residual calcified cartilage is largely unknown. The presence of elevated amounts of CS proteoglycans in the bones of osteopetrotic animals with defective osteoclasts was linked to the inability of these animals to resorb calcified cartilage . The functions of aggrecan in bone, if any, are also unknown. Because of its relatively low concentration, it seems likely that aggrecan affects mostly growth plate (cartilage) calcification, rather than having a direct effect on bone.
8.3.1.2
Versican
Versican (also known as PG-M in chickens, and CSPG2 in mammals) is another large CS proteoglycan related to aggrecan but found at relatively lower levels. Versican has been so named based on the large variety of isoforms in many types of extracellular matrices (versatility). The protein core, with a molecular weight of ~360 kDa, has a structure similar to that of aggrecan with the exception that it lacks the G2 domain. In addition, versican contains only 12–15 CS side chains (~45 kDa) in contrast to ~100 in aggrecan . There are four splice variants, some of which do not have GAG attachment site sequences in the protein core variant (reviewed in Ref. ). A report on embryonic rat bone development found that versican was expressed during mesenchymal condensation prior to cartilage formation , and during osteogenesis, where it was more abundant in woven bone than lamellar bone . The function of versican in cartilage and bone is largely unknown. Potentially, it may serve to occupy space destined to become bone, or as a bridge between the extracellular environment and the cell by binding to hyaluronic acid via the amino-terminal binding region and to molecules that have yet to be identified on the cell surface via the carboxy-terminal domain. In addition, versican stimulates chondrocyte proliferation . Because versican is degraded during matrix mineralization, the epidermal growth factor (EGF)–like sequences (G3 domain) may serve to stimulate proliferation of osteoprogenitors because EGF has been reported to stimulate proliferation of osteoblastic cells in vitro . With the exception of one study that showed increased versican degradation products accumulate in mineralizing osteoblast cultures, there are no other studies on the role of versican in mineralization of cartilage or bone.
8.3.2
Small leucine-rich repeat proteoglycans
In addition to aggrecan and versican, another family of proteoglycans is represented by a group whose protein core is characterized by a smaller size, and a leucine-rich repeat sequence that is ~20–30 amino acids in length (SLRPs) . The SLRP family has been subdivided into five classes based on their similarity in gene and amino acid structures . In bone and/or cartilage, the class I members include DCN (decorin), BGN (biglycan), ASPN (asporin), and ECM2; the class II members include FMOD (fibromodulin), KERA (keratocan), LUM (lumican), PRELP, and OMD (osteomodulin/osteoadherin); the class III members are EPYC (epiphycan/PG-Lb), OGN (osteoglycin/mimecan); the class IV members include CHAD (chondroadherin); and the class V members are PODN (podocan) and PODNL1 (podocan-like proteoglycan1) . Although SLRPs are highly homologous, they exhibit distinctively different patterns of posttranslational modifications (glycosylation and addition of GAG chains), expression, and tissue localization, indicative of divergent functions within these tissues. We will only discuss those that are relevant for cartilage calcification or bone mineralization in the following sections.
8.3.3
Class I small leucine-rich repeat proteoglycans associated with mineralization
8.3.3.1
Decorin
Decorin, so named for its ability to bind to and “decorate” collagen fibrils, has a core protein of ~38 kDa, which includes 10 leucine-rich repeat sequences. Although there are three potential GAG attachment sites, generally only one is utilized for the attachment of a single GAG ( Fig. 8.5 ). The length of the chain, and its extent of sulfation varies as a function of tissue type and age . Decorin is synthesized with a propeptide that is cleaved in bone and other tissues by the enzyme, BMP1 (Tolloid-related BMP1) . Decorin, like many of the other SLRPs, binds to and regulates collagen fibrillogenesis; however, only decorin can faithfully recapitulate the native superfibrillar organization of type I collagen in vitro . In bone the proposed functions of decorin are the regulation of collagen fibril diameter and fibril orientation, and possibly the prevention of premature osteoid calcification. Targeted disruption of the decorin gene results primarily in skin laxity and fragility in mice, whereas disruption of the biglycan gene (discussed next) results in reduced skeletal growth and bone mass leading to generalized osteopenia . Moreover, the decorin and biglycan double knockout mice have additive deficiency in dermis and synergistic effects in bone, and ultrastructural analysis of these mice reveals a complete loss of the basic fibril geometry with the emergence of marked “serrated fibril” morphology . The decorin/biglycan double knockout mouse also exhibits a dramatic decrease in cortical and trabecular bone volume, and bone mineral density . There is also a decreased expression of decorin in some patients with OI , in which abnormal mineral deposition has been detected outside the collagen matrix. A role for decorin in matrix mineralization by modulating collagen assembly is suggested by in vitro studies .
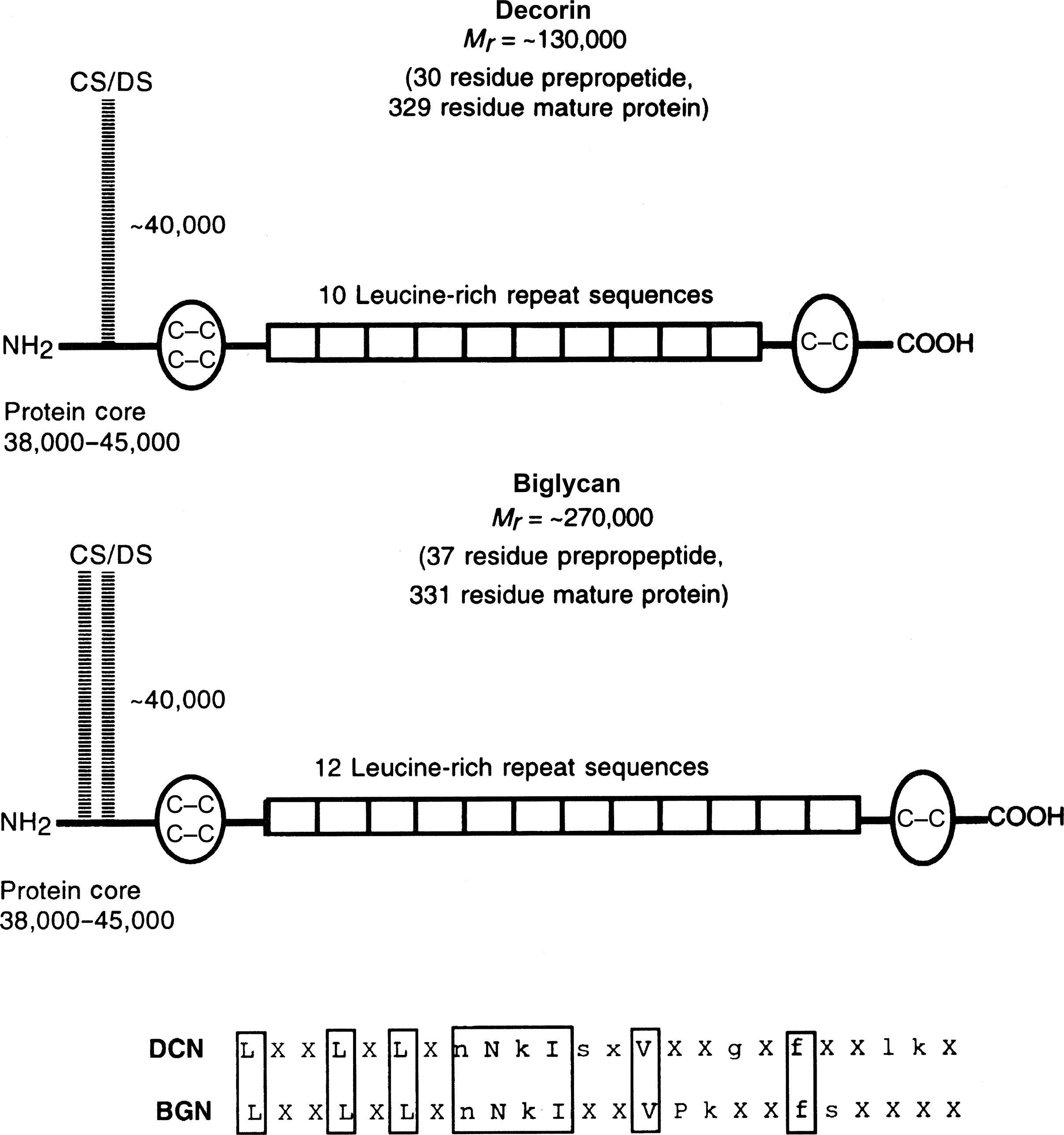
8.3.3.2
Biglycan
Biglycan is another small proteoglycan present in both cartilage and bone that is highly homologous to decorin ( Fig. 8.5 ). This SLRP has been found to modulate Wnt signaling and BMP2 activity in osteogenic cells. The functions of biglycan in cartilage and bone mineralization remain to be determined. In solution, biglycan at low concentrations can promote apatite formation, whereas at higher concentrations, it inhibits the growth and proliferation of mineral crystals . These effects appear to be due to the highly specific high-affinity binding of biglycan to apatite. Compared to the decorin knockout mice, the biglycan knockout mice have similar structural abnormalities in collagen fibrils but with a more serious impact in bone than in dermis . In addition, the biglycan knockout mice have shorter femora, decreased bone density, and fail to achieve peak bone mass compared with controls. The mineral within these bones has increased crystal size relative to wild-type controls , also indicating an inhibitory role of this protein. However, the low amount of biglycan present in bone matrix relative to other mineral nucleators and its absence from bone collagen fibrils suggests that its primary function may not be directly related to mineral deposition in bone.
8.3.3.3
Asporin
Asporin, the third member of the type I SLRP family present in bone, cartilage, and the periodontal ligament (all mineralizing tissues), was so named because of the unique aspartic acid repeat at the N terminus of the mature protein . This N terminus prevents asporin from being classified as a true proteoglycan based on the fact that there is no GAG attachment site. Asporin, like decorin, binds to type I collagen, and they compete with one another for the same binding site . Of the three class I SLRPs, only asporin binds calcium and increases calcium uptake into osteoblastic tissue culture . Interestingly, all three of the class I SLRPs, along with a class II SLRP, fibromodulin, have been shown to bind to TGF-β and sequester it within the matrix, thereby decreasing its activity and effects on osteogenic cells (reviewed in Ref. ).
8.3.4
Class II small leucine-rich repeat proteoglycans associated with mineralization
8.3.4.1
Fibromodulin
Fibromodulin is found predominantly in articular cartilage but also is present in bone. The intact protein is ~59 kDa, and the core protein shares a high homology with decorin and biglycan but bears KS GAG chains linked to asparaginyl residues rather than CS or DS linked to serinyl/threoninyl residues. Decorin and fibromodulin are the most active collagen-binding proteins in cartilage and bone, reportedly binding to completely different regions on collagen fibrils . Fibromodulin interacts with triple-helical types I and II collagens . In cartilage the amount of fibromodulin correlates with the size of collagen fibrils . Fibromodulin knockout mice have undermineralized bones and teeth at young ages but show increased dentinogenesis with increasing age , revealing a pleomorphic role of fibromodulin in mineralization as a function of age. Mice that are deficient in both fibromodulin and biglycan exhibit early onset of osteoporosis, and like the biglycan deficient mice, also have ectopic calcification in tendons .
8.3.4.2
Osteomodulin, keratocan, and lumican
Osteomodulin/osteoadherin is a minor, leucine- and aspartic acid-rich KS proteoglycan found in the mineralized matrix of bone and dentin. It is synthesized by osteoblasts and odontoblasts. In cell culture, it promotes osteoblast mineralization , whereas knockdown decreases odontogenic differentiation and mineralized nodule formation . Keratocan is also expressed by osteoblasts, and the knockout mouse has impaired bone formation, decreased expression of bone-specific markers and impaired mineralization , implying a role in osteoblast function. Lastly, lumican is also expressed by osteoblasts , but to date, the knockout mice were not reported to have any bone phenotype .
8.3.5
Class III small leucine-rich repeat proteoglycans involved in mineralization
8.3.5.1
Epiphycan
Epiphycan is expressed during early development in the growth plate and articular cartilage. In its absence, in older mice, femurs are shorter due to abnormalities in the growth plate. They also show a loss of collagen. In aging mice the absence of epiphycan accelerates the development of osteoarthritis. The epiphycan/biglycan double knockout mouse has a more pronounced phenotype, suggesting some synergistic interaction between these two SLRPs .
8.3.5.2
Chondroadherin
Chondroadherin is composed of a 38 kDa core protein with a single KS GAG side chain. The chondroadherin deficient mouse has both a cartilage and bone phenotype. Deficiency leads to an expanded proliferative zone in the growth plate, and to an increase in osteoid, but a direct role in matrix mineralization has-yet to be determined. Deficient mice have decreases in numerous structural bone parameters, and mechanical testing reveals weaker bones .
8.4
Bone-enriched matrix proteins
In bone, the remaining matrix proteins are mainly composed of two major types: glycoproteins and γ-carboxyglutamic acid (Gla)-containing proteins. The most relevant and abundant glycoproteins are represented by tissue-nonspecific alkaline phosphatase (TNAP) (alkaline phosphatase), secreted phosphoprotein acidic and rich in cysteine (SPARC) (osteonectin), and the cell attachment proteins, which include but are not limited to the SIBLING (small integrin-binding ligand, N-linked glycoproteins) family and additional sialoproteins. Of the Gla-containing proteins, BGLAP (osteocalcin) is the major representative. These bone matrix proteins have divergent biochemical properties and play particular roles in the regulation of matrix mineralization.
8.4.1
Glycoproteins
This class of proteins is characterized by the covalent linkage of sugar moieties attached via asparaginyl or serinyl/threonyl residues. Collagen also contains another form of glycosylation (galactosyl-hydroxylysine and glucosyl-galactosyl-hydroxylysine), which is virtually specific to collagen. These glycoproteins, which include membrane-bound enzymes and matrix proteins, may be further modified by posttranslational sulfation and phosphorylation.
8.4.1.1
Alkaline phosphatase
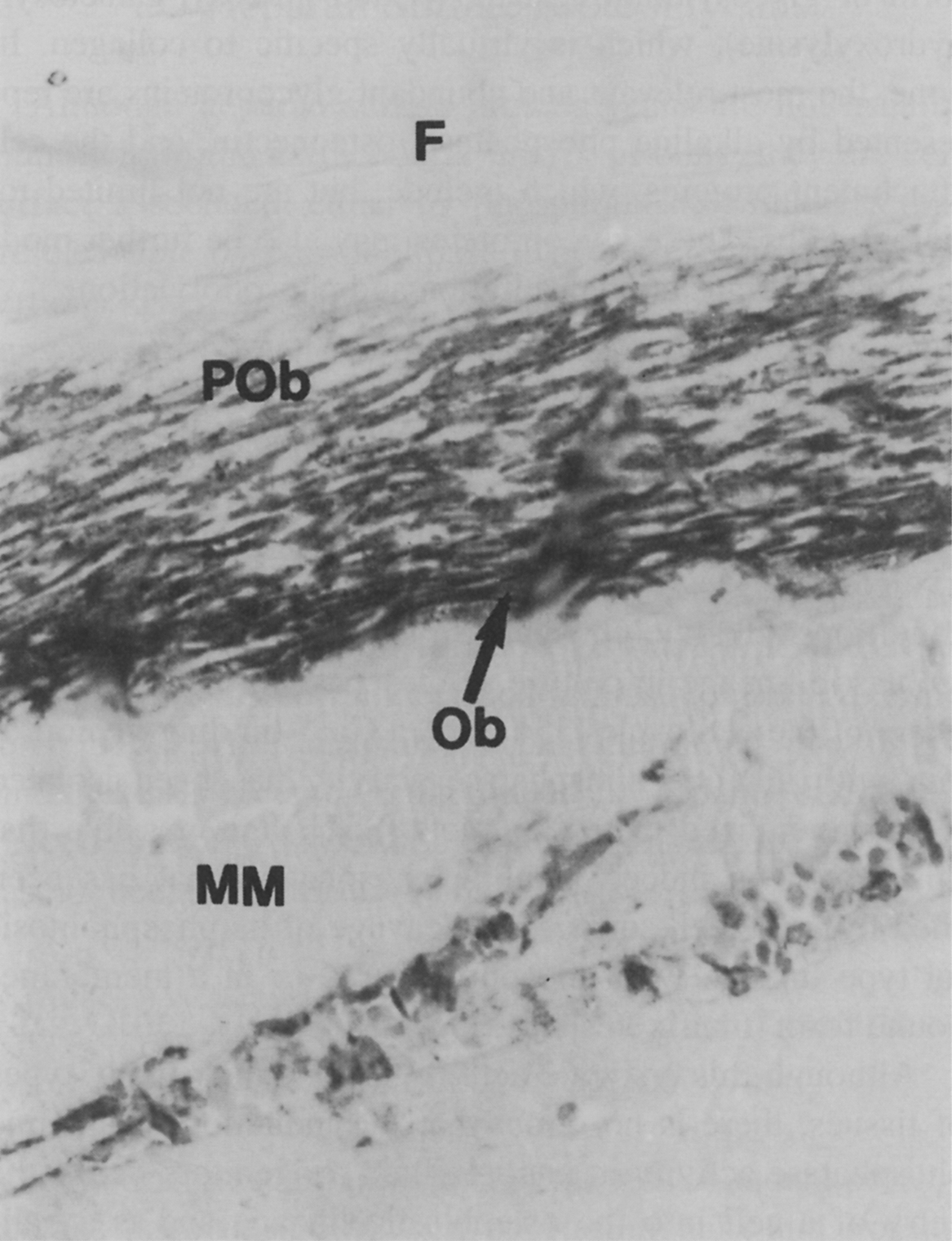
Although the enzymatic activity of alkaline phosphatase is shared by many types of tissues, there is no doubt that induction of alkaline phosphatase activity in uncommitted progenitors marks the entry of a cell into the osteogenic lineage and is a hallmark in bone formation. Alkaline phosphatase is not typically thought of as a matrix protein; however, studies indicate that alkaline phosphatase can be shed from the cell surface of osteogenic cells or secreted in a membrane-bound form (matrix vesicles) . Histological localization of alkaline phosphatase in developing human subperiosteal bone ( Fig. 8.6 ) marks its very specific expression by preosteoblasts and osteoblasts in areas that are destined to become new bone, whereas less expression was found in mineralized matrix , suggesting this enzyme as a marker for less mature, osteoprogenitors. Mice with null mutations for the tissue-nonspecific (bone/liver/kidney) alkaline phosphatase provide evidence of the importance of alkaline phosphatase for mineralization , showing increased osteoid and defective growth plate development. It is thought, based on studies with these knockout mice and studies of patients with hypophosphatasia, that alkaline phosphatase functions to regulate the pyrophosphate levels, while another enzyme, PHOSPHO1 is involved in the initiation of mineralization . Pyrophosphate is an inhibitor of mineralization that can be hydrolyzed to provide additional phosphate while removing a mineralization inhibitor. Alkaline phosphatase is the major enzyme carrying out this hydrolysis, although phosphate transport is regulated by a number of other enzymes. A recent study has also suggested that alkaline phosphatase and tartrate-resistant acid phosphatase may also play a role in dephosphorylating osteopontin, thereby inactivating its inhibitory action on mineralization .
8.4.1.2
Osteonectin
One of the first noncollagenous bone matrix proteins to be isolated and characterized was osteonectin . Osteonectin, also called SPARC, culture shock protein, or BM-40 (basement membrane tumor factor 40), is expressed in a number of tissues during development and by many cell types. In bone, osteonectin can constitute up to 15% of the noncollagenous protein depending on the developmental age and the animal species . Osteonectin has an apparent molecular weight of ~35 kDa without reduction of disulfide bonds and gel electrophoresis mobility ~40–46 kDa following reduction, indicative of intrachain disulfide bonds ( Fig. 8.7 ). Due to the nature of the amino acid composition and of the posttranslational modifications, osteonectin is acidic. Osteonectin may be differentially glycosylated and/or phosphorylated because there are at least two potential N -glycosylation sites that bear diantennary oligosaccharides (an intermediate between high mannose and complex-type oligosaccharides that contains variable amounts of sialic acid and fucose) .

Osteonectin is essential for the maintenance of bone mass and for balancing bone formation and resorption in response to parathyroid hormone. It promotes osteoblast differentiation and cell survival similar to other “matricellular proteins.” The matricellular proteins were so named in 1995 as “a group of modular, extracellular proteins whose functions are achieved by binding to matrix proteins as well as to cell surface receptors, or to other molecules such as cytokines and proteases, that interact in turn, with the cell surface” . These include osteonectin, the thrombospondin (TSP) family, the tenascin family, the CCN family (small secreted cysteine-rich proteins that function as signaling molecules ), and the SIBLING family . All of these are involved in responses to injury and stress, and the pathogenesis of several chronic diseases of aging. Osteonectin’s effects are tissue specific, including regulation of procollagen processing, collagen fibril assembly (skin and bone), and modification of cell shape, migration, proliferation, differentiation, survival, and modulation of cell signaling . Osteonectin-null mice are osteopenic, show increased marrow adiposity, and have cortical bone with decreased mechanical properties and matrix quality . The osteonectin knockout was shown to have bones with increased mineral content and crystallinity and increased collagen maturity at all sites, based on FTIR microspectroscopy and imaging . The presence of a low turnover form of osteopenia suggested by these data was confirmed . The spines of mice with targeted deletion of osteonectin similarly showed sclerosis and premature end plate calcification . Recently, mutations in osteonectin in the collagen-binding pocket region have been associated with a form of OI. Other mutations have been associated with idiopathic osteoporosis supporting the notion that osteonectin plays a role in ECM assembly and architecture (reviewed in Ref. ).
8.4.1.3
RGD-containing glycoproteins
In bone matrix, there are a number of glycoproteins that include the amino acid sequence, RGD. These RGD sequences can be recognized by cell surface receptors as a “cell attachment sequence” that bridges the attachment between extracellular matrix and cells and organizes the cells in matrix. These RGD-containing proteins expressed in bones and teeth include collagen (described previously), TSPs, FN (fibronectin), VN (vitronectin), and the SIBLINGs. The SIBLINGs are a family of genes, including SPP1 (osteopontin), bone sialoprotein, DMP1 (dentin matrix protein 1), DSPP (dentin sialophosphoprotein), MEPE (matrix extracellular phosphoglycoprotein), and ENAM (enamelin) .
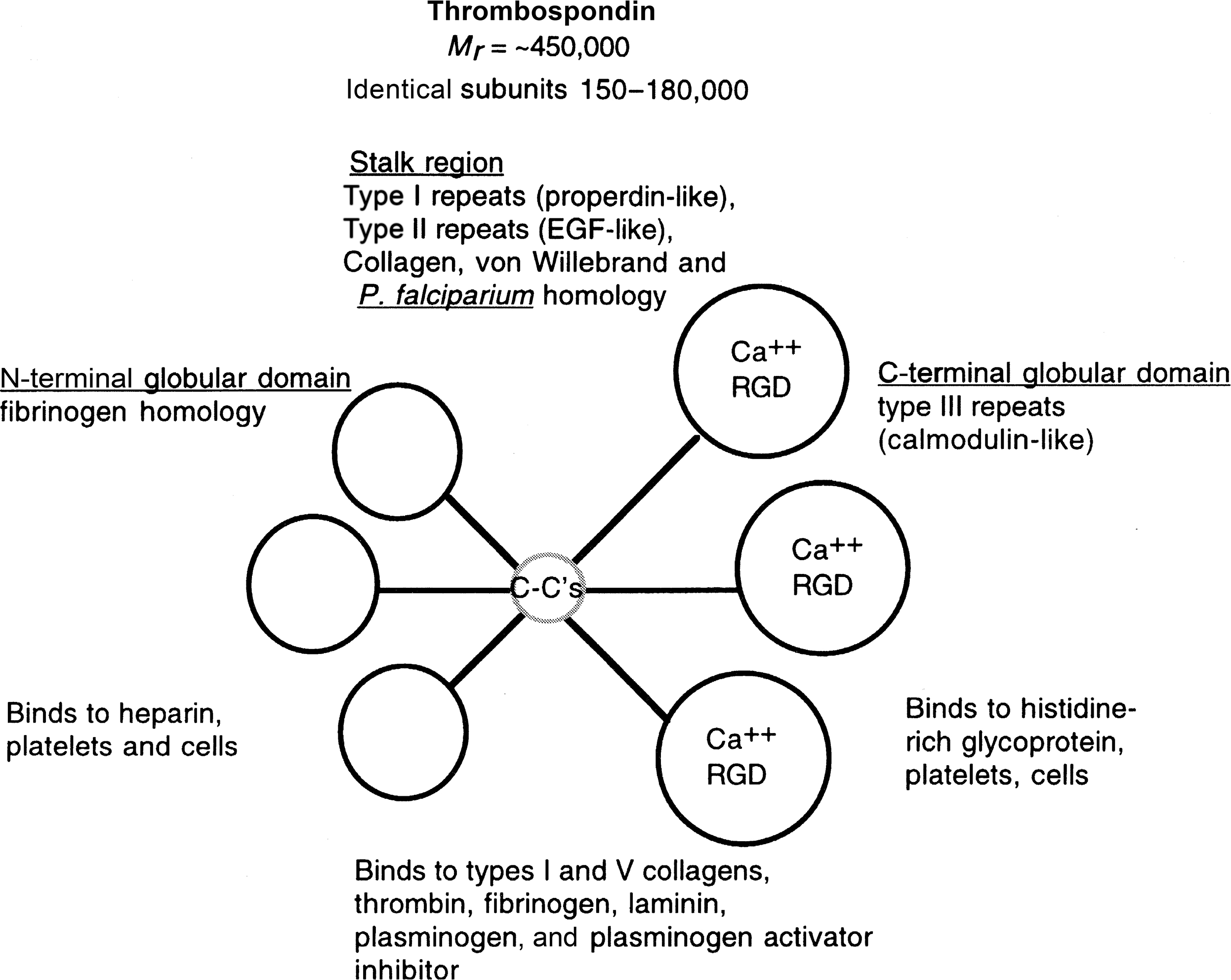
a. TSP(s) . This class of glycoproteins contains five members (TSP1–4, and TSP5, also known as COMP), and all are expressed in a large number of connective tissues, including bone at varying levels . These are complex modular glycoproteins that share structural homology due to so-called TSP repeats ( Fig. 8.8 ). The R-spondin family also has these repeats . TSP2 regulates osteoblasts and affects bone mass and geometry, and response to injury. Compound knockout mice of TSP1 and 2 have altered craniofacial development. The TSP1/3/5 triple knockout mice display growth plate abnormalities. R-spondin 2 knockout mice also have skeletal defects. These combined data show a role for TSP family molecules in bone development and turnover. TSP1 inhibits mineralization whereas TSP2 promotes it, and TSP5/COMP appears to be a matrix organizer in cartilage .