(1)
Institute of Oncology “Prof. Ion Chiricuță”, Nuclear Medicine & Endocrine Tumors, Cluj-Napoca, Romania
Nature does nothing uselessly.
Aristotle (Antique philosopher 384–322 B.C.E)
8.1 Anatomy of the Parathyroid Glands
The parathyroid glands are located on the posterior surface of the thyroid gland.
These four glands produce the parathyroid hormone (PTH), which helps to maintain calcium homeostasis by acting on the renal tubule as well as calcium stores in the skeletal system and by acting indirectly on the gastrointestinal tract through the activation of vitamin D (Beierwaltes 1991).
The parathyroid glands have a distinct, encapsulated, smooth surface that differs from the thyroid gland, which has a more lobular surface.
The superior parathyroid glands are most commonly located in the posterior-lateral aspect of the superior pole of the thyroid gland at the cricothyroid cartilage junction. They are most commonly found 1 cm above the intersection of the inferior thyroid artery and the recurrent laryngeal nerve.
The inferior parathyroid glands are more variable in location and are most commonly found near the lower pole of the thyroid.
8.1.1 Embryology
The parathyroid glands develop from the endoderm of the third and fourth pharyngeal pouches. The inferior parathyroid glands are derived from the dorsal part of the third pharyngeal pouch, and the thymus arises from the ventral part of the third pharyngeal pouch. As the inferior parathyroid glands and the thymus migrate together toward the mediastinum, they eventually separate. In most cases, the inferior parathyroid glands become localized near the inferior poles of the thyroid, and the thymus continues to migrate toward the mediastinum.
The superior parathyroid glands are derived from the fourth pharyngeal pouch. The superior parathyroid glands migrate a shorter distance than the inferior glands, which results in a relatively more constant location in the neck.
8.1.2 Parathyroid Vascularity
The inferior parathyroid glands that descend into the anterior mediastinum are usually vascularized by the inferior thyroid artery. If a parathyroid is positioned low in the mediastinum, a thymus branch of the internal thoracic artery or even a direct branch of the aortic arch may supply it.
The superior parathyroid gland is usually supplied by the inferior thyroid artery or by an anastomotic branch between the inferior thyroid and the superior thyroid artery.
The location of the inferior parathyroid glands exhibits a greater degree of variability than the superior parathyroid glands. If the inferior glands fail to separate or separation from the thymus is delayed during their descent, the inferior glands may have ectopic locations within the superior mediastinum.
In rare cases, there is possible to have an intrathyroid localization. After adhering to the thyroid capsule, the parathyroid gland may actually enter the thyroid gland and embed there rather than remaining outside the gland. This situation results in an intrathyroidal parathyroid gland.
8.2 Physiology and Pathophysiology of the Parathyroid Glands
The main role of the parathyroid glands is to produce the parathyroid hormone (PTH). The secretion of PTH is stimulated by low serum calcium concentration and inhibited by high serum calcium. The role of phosphate is not yet clear: high concentration stimulates secretion of PTH; this effect may also be an indirect consequence of the high phosphate levels, causing a reduction of serum calcium concentration (Arnold et al. 2002; Beierwaltes 1991).
The two forms of vitamin D (D2-ergocalciferol and D3-cholecalciferol) are both activated to the calcitriol form. The calcitriol has an important role in the long-term regulation of plasma calcium levels and therefore regulates the PTH production.
PTH acts on the kidney and the bone as targeted organs.
The effects of PTH on the kidney:
Increases the reabsorption of calcium from the urine
Increases the excretion of phosphates
Increases the expression of the enzyme 1α-hydroxylase, which activates vitamin D
The effects of PTH on the bone:
High levels increase bone resorption by stimulating the activity of osteoclasts.
Low levels increase bone formation by stimulating the activity of osteoblasts.
8.2.1 Pathophysiology
More than 80% of the patients are asymptomatic or have nonspecific symptoms (AACE/AAES Task Force 2005), while less than 15% of the patients are presenting renal stones.
Primary hyperparathyroidism results from a solitary adenoma in over 80% of the cases. Diffuse hyperplasia occurs less frequently and very rarely, parathyroid carcinoma accounting for about 4%.
The treatment is surgical with a high success rate of 90–95%, even if preoperative localization procedures fail sometimes in identifying the lesion. Recurrent and persistent hyperparathyroidism is usually related to aberrant or ectopically located glands or recurrent hyperplasia. Re-exploration is technically difficult with a higher morbidity and poorer success rate than initial surgery. Diffuse hyperplasia accounts for approximately 15% of the cases of primary hyperparathyroidism, and a substantial proportion of these may occur in association with the multiple endocrine neoplasias (MEN syndromes).
Secondary hyperparathyroidism is associated with chronic renal failure being also related to diffuse hyperplasia; it may require surgical therapy due to progressive bone disease. Secondary hyperparathyroidism is consequent to a chronic hypocalcemic condition that can be caused also by gastrointestinal malabsorption, dietary rickets, and ingestion of drugs, like phenytoin, phenobarbital, and laxative, which generate a decreased intestinal absorption of calcium. The continuous stimulus to produce and to secrete PTH results in parathyroid gland hyperplasia. Secondary hyperparathyroidism is a frequent and serious complication in hemodialysis patients. Because all the parathyroid tissue is stimulated, the presence of unsuspected supernumerary glands has major impact in terms of surgical failure.
Tertiary hyperparathyroidism occurs during dialysis. Tertiary hyperparathyroidism is a condition where parathyroid hyperplasia, secondary to chronic hypocalcemia, becomes autonomous with development of hypercalcemia. This condition generally does not regress after the correction of the underlying cause that generated chronic hypocalcemia. Usually, in tertiary hyperparathyroidism, we find asymmetrical parathyroid gland hyperplasia. Tertiary hyperparathyroidism is also used to designate hyperparathyroidism that persists or develops after renal transplantation.
The Parathyroid Center from Tampa General Hospital, Florida, USA, led by one of the most experienced parathyroid surgeons, Professor Jim Norman, has created a brief description of the main features of pathologic parathyroid gland known as: “The ten parathyroid rules of Norman” (Norman 1998b).
8.2.2 The Ten Parathyroid Rules of Norman
- 1.
There are no drugs that will treat parathyroid disease.
- 2.
Most (>95%) parathyroid patients have symptoms.
- 3.
Symptoms of parathyroid disease do not correlate with the level of calcium in the blood.
- 4.
All patients with parathyroid disease have fluctuating calcium levels and PTH levels.
- 5.
All patients with hyperparathyroidism will develop osteoporosis.
- 6.
The drugs for osteoporosis do not treat parathyroid disease.
- 7.
Parathyroid disease will get worse with time in all patients.
- 8.
There is only one treatment for primary hyperparathyroidism: surgery.
- 9.
Most parathyroid patients can be cured with a minimal operation.
- 10.
The success rate and complication rate for parathyroid surgery are very dependent upon the surgeon’s experience.
8.3 Diagnosis of the Parathyroid Glands
8.3.1 Clinical Examination
The parathyroid glands are less accessible to clinical inspection than is the thyroid gland. Despite this, the very specific determination of the parathyroid hormone (PTH) gives the physicians the possibility to find the suggestive elements for parathyroid disorders, in patient’s history.
8.3.1.1 History
The parathyroid glands require a careful anamnestic interview with the aim to discover signs and symptoms that might be related to hypo- or hypercalcemia (Silverberg et al. 1999). Parathyroid pathology may have genetic determinism; primary hyperparathyroidism is possible to occur in association with other endocrine diseases, in the so-called multiple endocrine neoplasias (MENs). The history is one of the first steps in the patient evaluation.
Due to severe increasing of nodular goiter and extensive thyroid surgery, the pathology of accidental parathyroidectomy is frequent nowadays. The hypocalcemia mainly due to deficient intake or surgery complications does not represent a topic in the present chapter. The area of interest will be the identification of parathyroid glands responsive for primary hyperparathyroidism.
History will bring information related to:
Age (elder patients need special attention, firstly because of more frequent bone pathologies)
Gender (female are more frequently affected by osteoporosis, and the differential diagnosis to parathyroid involvement is crucial for the evolution of the disease)
Signs/symptoms of hyper-/hypothyroidism; the thyroid pathology may be associated
Rapid evolution of osteoporosis severity
Digestive disorders due to hypercalcemia (ulcers)
Kidney lithiasis and gallbladder lithiasis occurring in familial aggregation
History may report association of parathyroid pathology into other syndromes:
Found to have association with medullary thyroid cancer (MTC) in MEN II syndrome (parathyroid adenoma, MTC, and pheochromocytoma)
Pituitary adenoma, parathyroid adenoma, and pancreatic islet cell tumors (MEN I—Wermer syndrome)
History elements suggestive for malignancy:
Rapid progressive enlargement of cervical area
Hoarseness
Dysphagia
Dyspnea
Lymph nodes
Familial history of other thyroid cancers
8.3.1.2 Clinical Examination
The situation of parathyroid glands in the posterior face of the thyroid gland makes the inspection almost impossible. The lymph node palpation of the neck should be carefully done undergoing the known pathways of lymphatic drainage: lateral-cervical, supraclavicular, and submental. This is important for the situation of associated thyroid tumors; parathyroid cancer is very unusual, so the signs determined by this pathology are very rare.
The inspection will focus mainly on the signs and symptoms produced by hypercalcemia or by other neuroendocrine mediators secreted by the associated tumors.
General signs:
Bone pain with different degrees of impairment of motion and bone abnormalities
Diarrhea if it is in association with carcinoid tumors
Anxiety, depression, lethargy, and other mental disorders
Weight loss
Muscle weakness
Extreme fatigue
Other symptoms dictated by associated neuroendocrine tumors
All these signs and symptoms come to design a clinical, very severe presentation that may very seriously affect the patient’s status and which frequently is discovered late.
8.3.2 Laboratory Serum Testing
8.3.2.1 Calcium, Phosphorus, and Vitamin D
With primary hyperparathyroidism, people will generally have high calcium and high PTH levels, while phosphorus levels are often low. Secondary hyperparathyroidism is usually due to kidney failure, and phosphorus may not be excreted efficiently, disrupting its balance with calcium. Kidney disease may also make those affected unable to produce the active form of vitamin D, and this means that they are unable to properly absorb calcium from the diet. As phosphorus levels build up and calcium levels fall, PTH is secreted. Secondary hyperparathyroidism can also be caused by any other condition that causes low calcium, such as malabsorption of calcium due to intestinal disease and vitamin D deficiency.
8.3.2.2 Parathyroid Hormone
PTH is ordered to help diagnose the reason for a low or high calcium level and to help distinguish between parathyroid-related and non-parathyroid-related causes. A doctor will evaluate both calcium and PTH results together to determine whether the levels are appropriate and are in balance as they should be.
Calcium-PTH relationship (AACE/AAES 2005)
If calcium levels are low and PTH levels are high, the parathyroid glands respond, as they should, and produce appropriate amounts of PTH. A low calcium level must be investigated by further measurement of vitamin D, phosphorus, and magnesium levels.
If calcium levels are low and PTH levels are normal or low, then PTH is not responding, and the person tested probably has hypoparathyroidism. It may be due to a variety of conditions and may be persistent, progressive, or transient. Those affected will generally have low PTH levels, low calcium levels, and high phosphorus levels.
If calcium levels are high and PTH levels are high, then the parathyroid glands are producing inappropriate amounts of PTH. This is the current presentation of hyperparathyroidism.
If calcium levels are high and PTH levels are low, then the parathyroid glands are responding properly, but the doctor is likely to perform further investigations to check for non-parathyroid-related reasons for the elevated calcium.
PTH levels will vary during the day, peaking at about 2 a.m. Specimens are usually drawn about 8 a.m. Drugs that may increase PTH levels include phosphates, anticonvulsants, steroids, isoniazid, lithium, etc. PTH can be measured in the blood in several different forms, intact PTH, N-terminal PTH, mid-molecule PTH, and C-terminal PTH, and different tests are used in different clinical situations. The average PTH level is of 10–60 ng/L.
Insulin, glucagon, calcitonin, prolactin, gastrin, chromogranin A, serotonin, metanephrine, etc., all these hormones may be determined in the serum level having a contribution in the clinical status of parathyroid association in other endocrinopathies.
8.3.3 Imaging Tests
8.3.3.1 Plain Films
In the parathyroid pathology are indicated hands films and other bone radiographies for the diagnosis of bone abnormalities
8.3.3.2 Dual-Energy X-Ray Absorptiometry
DEXA may be useful in assessing chronic bone mineral loss. Significant bone loss—absolute (T score) and age-related (Z score)—may be an indicator for parathyroid surgery, in primary hyperparathyroidism, even in the absence of other clinical symptoms or marked hypercalcemia.
8.3.3.3 CT and MRI
The association with different other endocrine tumors makes the imagistic tests for parathyroid to be complex:
Parathyroid glands: neither CT nor MRI are imaging tests of first line, due to the low sensitivity comparing with nuclear tests.
Thyroid gland: ultrasound and FNAB in case of suspicious MTC.
Pituitary tumors: Magnetic resonance imaging (MRI), with attention to the sella turcica region, is the test of choice when biochemical evidence of pituitary disease is found. Some tumors may not be functioning; however, asymptomatic patients should also be screened regularly (every 3–5 years).
Gastrinomas: Somatostatin receptor scintigraphy is the imaging procedure of choice for gastrinomas. Its sensitivity range is 70–90%. Somatostatin receptor scintigraphy can be enhanced by selective arterial secretagogue test with secretin or calcium infusion (in 10% of the cases of gastrinomas secretin, it is not diagnostically useful). Operative intervention should be preceded by a computed tomography (CT) or MRI scan. Endoscopic ultrasonography detects tumors in the pancreatic head but rarely in the duodenal wall. It is more sensitive than the CT or the transabdominal ultrasound.
Insulinomas: CT scan and MRI are recommended first. Somatostatin receptor scintigraphy findings may be positive in up to 50% of patients with insulinomas. It is best used in conjunction with single-photon emission CT (SPECT). Endoscopic ultrasonograph has a reported detection sensitivity of up to 94%.
8.3.3.4 Nuclear Medicine Tests
Thallium-201 chloride was the first agent used to successfully image the parathyroid glands in the 1980s. Tc-99m MIBI (Chudzinski et al. 2005; Mitchell 1996; Lavely et al. 2007) was first described for parathyroid localization involving the subtraction technique using I-123 to outline the thyroid. I-123 has the advantage of being a compound that is both trapped and organified by the thyroid. However, it is expensive and normally requires a delay of some hours between administration and imaging, with a lengthening of the entire procedure. The uptake of Tc-99m MIBI per gram of parathyroid tissue was lower than for thallium, but the ratio between the parathyroid and thyroid tissue was higher. Tc-99m tetrofosmin has also been investigated for imaging parathyroid tissue since it is a similar class of radiopharmaceutical as Tc-99m MIBI. Positron emission tomography (PET) tracers have met variable success. 18-Fluoro-2-deoxy-d-glucose (FDG) has been found useful by some authors for the identification of parathyroid adenomas, although others have not had this success. C-11 methionine seems promising although there is still limited experience. In the latest years, PET/CT with F18-choline showed interesting results in identifying parathyroid adenoma. Nevertheless, it is useful when there are problems in the identification of a parathyroid site with conventional scintigraphy.
Thallium, MIBI, and tetrofosmin are all cardiac imaging agents. In the neck, they are taken up by the salivary tissue as well as by the thyroid and parathyroid glands. In the chest, the heart is clearly visualized, but the mediastinum should be clear of focal uptake with only a low-grade blood pool depending on the imaging time. For the PET tracers, FDG has low-grade uptake in the mediastinum as a result of blood pool and can have low-grade focal uptake in lymph nodes of benign cause.
8.3.3.5 Parathyroid Scintigraphy Dual-Tracer Technique (Subtraction Scanning) Tc-99m Pt/I-123 and Tc-99m MIBI/Tc-99m Tetrofosmin/Tl-201 Chloride
Radiopharmaceuticals:
I—Technetium-99m pertechnetate (Tc-99m Pt) or I-123 for thyroid phase
II—Technetium-99m MIBI or Tc-99m tetrofosmin or thallium-201 chloride
Principle:
The examination is based on the differential washout of Tc-99m MIBI or Tc-99m tetrofosmin or thallium-201 chloride from the thyroid tissue compared with abnormal parathyroid tissue. The rate of washout from the abnormal parathyroid tissue, such as parathyroid adenoma, is much slower than that of the normal thyroid tissue (Hindie et al. 2009).
The distributions of the two tracers can be visually compared. The thyroid scan can be digitally subtracted from the parathyroid scan; removing the thyroid activity enhances the visualization of the parathyroid tissue. Lower sensitivity of Tl-201 together with higher radiation dose compared to Tc-99m MIBI made its use for parathyroid scanning less frequent.
Tc-99m tetrofosmin is an alternative to Tc-99m MIBI for parathyroid subtraction scanning. Absence of differential washout in the thyroid and parathyroid has not allowed its use as a single agent for double-phase study. However, because only early imaging is required with subtraction imaging, Tc-99m tetrofosmin can be an appropriate tracer.
Indications:
Diagnosis of parathyroid adenomas.
To detect recurrent or persistent disease both in primary hyperparathyroidism (PHPT) and secondary hyperparathyroidism.
To improve results of initial surgery in PHPT.
To select patients with PHPT for unilateral surgery or focused surgery, instead of the conventional bilateral neck exploration.
The use of Tc-99m MIBI scanning before initial surgery of secondary hyperparathyroidism is controversial.
Technique:
Patient preparation:
No need for fasting.
No need for thyroid hormone withdrawal in case of Tc-99m, but in case of I-123, attention to all blocking agents.
Influence of other drugs: calcium intake, drugs for osteoporosis must be interrupted at least 1 week before examination.
Attention to breastfeeding patients, children, and potential fetal exposure.
The administered activity is of 370–555 MBq (10–15 mCi)/patient of Tc-99m MIBI.
The average activity of I-123 is of 15 MBq (400 μCi).
The activity of the used Tc-99m pertechnetate depends on the protocol: about 60–100 MBq is used if the protocol starts by thyroid imaging, and 150 MBq is given if thyroid imaging is done at the end of the Tc-99m MIBI procedure.
185 MBq (5 mCi) of Tc-99m tetrofosmin or Tl-201 chloride.
Dose calibration.
Injected I.V.
Procedure for simultaneous dual–tracer Tc–99m MIBI and I–123 scanning (planar)
I-123 is given.
Two hours later, the patient is placed under the gamma camera, and the Tc-99m MIBI is injected.
Images are acquired simultaneously using appropriate windows without energy overlap.
Imaging can start 3–5 min after Tc-99m MIBI injection with a broad field of view of the neck and mediastinum extending from the submandibular salivary glands to the upper part of the myocardium to ensure detection of ectopic glands.
Digital data are acquired in a 256 × 256 matrix using a LEHR collimator.
The acquisition procedure may also use a pinhole collimator.
An image analysis computer program is used to subtract a progressively increasing percentage of the I-123 image from the Tc-99m MIBI.
Delayed images (2–4 h postinjection) are not useful and do not increase the sensitivity of the subtraction protocol.
Additional image acquisition may be useful.
When ectopic uptake is observed (in mediastinum or submandibular), an additional SPECT acquisition is useful.
SPECT/CT images should be used whenever is possible, due to higher sensitivity.
Procedure for double-tracer Tc-99m-pertechnetate and Tc-99m MIBI with successive acquisition
A subtraction scan based on sequential image acquisition of these two tracers can be carried out in many different ways:
- 1.
The patient is injected I.V. with 185 MBq Tc-99m Pt.
After 20 min, the thyroid image is acquired.
At the end, keeping the patient in the same position, 300 MBq of Tc-99m MIBI is administrated I.V., and a 20 min dynamic acquisition is performed.
This protocol has good sensitivity and specificity, but it has a drawback: high count rates from the thyroid gland do not allow, after the subtraction, the identification of a small parathyroid hyperfunctioning gland located behind the thyroid.
- 2.
Tc-99m Pt 40–60 MBq I.V.
Twenty minutes after the injection, a 10 min image is obtained.
Then, without moving the patient, 370–600 MBq of Tc-99m MIBI is injected.
Five minutes after the injection, a pinhole image of the neck is recorded for 15 min (or a 20/35 min dynamic acquisition).
- 3.
The technique suggested by Rubello et al. (2003). This procedure consists in the oral administration of 400 mg potassium perchlorate immediately before starting the acquisition of the thyroid scan, with the aim of inducing rapid Tc-99m Pt washout from the thyroid and reducing its interference on the Tc-99m MIBI.
150–200 MBq of Tc-99m Pt and 550–600 MBq of Tc-99m MIBI are injected.
A matrix size of 128 × 128 is adequate.
A large field of view image with parallel hole collimator is always necessary to detect aberrant parathyroids and should include the submandibular salivary glands and the upper part of the myocardium.
Realignment of images is sometimes needed to correct the patient motion between the two sets of images.
Clinical applications:
Diagnosis of hyperparathyroidism
Necessary additional examinations:
Clinical examination; remember to discuss and to examine the patient BEFORE the injection, in order to limit the hazardous radiation exposure.
Thyroid and parathyroid ultrasound.
Serologic tests: serum level of PTH, serum and urinary level of calcium and phosphates, and alkaline phosphate.
Comments:
The place of scintigraphy in the assessment of hyperparathyroidism is being largely controversial, related to the finding of the best radiotracer and to the important limitation of other imagistic and structural diagnostic methods.
To reach a high sensitivity in detecting multiglandular disease with subtraction techniques, the degree of subtraction should be monitored carefully. Over-subtraction can easily delete additional foci and provide a wrong image suggestive of a single adenoma.
The use of SPECT/CT improved specificity, but unfortunately could not improve sensitivity. The role of dual-tracer SPECT/CT should be investigated in recurrent hyperparathyroidism as it allows correlation with morphological information, but clearly, low sensitivity in the thyroid bed area compared to pinhole subtraction imaging precludes its routine use before the first operation.
Reports:
The reports respect the general format of the department with all the identification data of the patient, the institution, and the physician; the technical data related to the radiopharmaceutical, the dose used, the type of gamma camera, the acquisition data, and the estimation of absorbed dose due to this examination.
The report will include the positive diagnosis of parathyroid adenoma and the lateralization and if it is possible the most accurate localization.
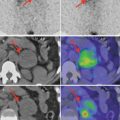
Normal image of parathyroid scan in dual-phase-tracer technique: adding the subtraction procedure, if there is no pathology in the parathyroid area, the glands will not appear in the image (Fig. 8.1).
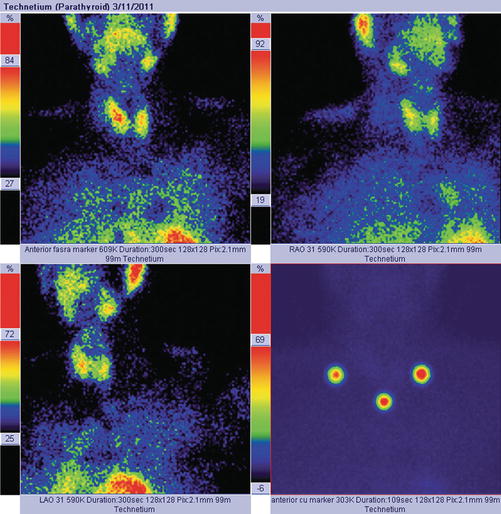

Fig. 8.1
Normal dual-tracer parathyroid scan with subtraction. The image with markers (right bottom) shows the thyroid “clean” area, with no tracer uptake
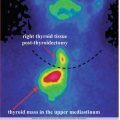
Case 1: Inferior Right Parathyroid Adenoma
History:
A 44-year-old female, with previous history of reno-ureteral lithiasis and gastric ulcer, is referred.
Clinical examination:
Symptoms: extreme fatigue, muscle pain and weakness, insomnia, and weight loss of 4 kg in 2 months
The neck examination does not reveal any pathological findings. No clinical evidence of lymph nodes.
Examinations:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree