Over the past 50 years, the National Surgical Adjuvant Breast and Bowel Project (NSABP) has made significant contributions in reducing the extent of surgical resection and in improving the outcome of patients with early-stage breast cancer through the conduct of large, randomized clinical trials evaluating various aspects of local and systemic therapy. Some of these trials have been instrumental in establishing new standards of care in the locoregional and adjuvant systemic therapy for these patients. The rationale, design, and updated results from those pivotal trials are reviewed in this chapter. In addition, the design and rationale for newer trials put forth by the NSABP and other major cooperative groups in an attempt to refine several aspects of adjuvant therapy and introduce new promising drugs are also reviewed. Finally, current and future research directions of the NSABP in the context of other developments in the surgical and adjuvant breast cancer therapy are discussed.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) has been instrumental in changing the paradigm of the surgical management of both invasive and noninvasive breast cancer that was founded on Halstedian principles of tumor growth and dissemination. Several randomized trials (B-04, B-06, B-17) have demonstrated that the extent of local therapy is not paramount to patient’s survival.1-3 As a result of these trials as well as those conducted by other groups,4,5 disfiguring operations developed a century ago, such as radical mastectomy, have now been replaced in the majority of cases by the more cosmetically acceptable and less function-impairing lumpectomy.
The NSABP B-04 trial was set up as 2 companion trials conducted in parallel, one for patients with clinically node-negative breast cancer and the other for those with clinically node-positive disease. Since radical mastectomy was the standard of care at that time, this operation was included as the control arm for both trials. One thousand seventy-nine patients with clinically node-negative disease were randomized to radical mastectomy (362 patients), total mastectomy plus local-regional/axillary irradiation (352 patients), or total mastectomy alone, with no targeted axillary treatment (365 patients). For patients presenting with clinically suspicious disease in the axilla, 586 patients were randomized to radical mastectomy (292 patients) or total mastectomy plus radiation (294 patients). The most recent update from the B-04 trial after 25 years of follow-up6 continues to demonstrate no significant differences in long-term outcome between clinically node-negative patients who received radical mastectomy and those who received total mastectomy with or without nodal irradiation, or between clinically node-positive patients who received radical mastectomy and those who received total mastectomy with nodal irradiation. Among women with clinically negative nodes, the hazard ratio for death among those who were treated with total mastectomy and irradiation as compared with those who underwent radical mastectomy was 1.08 (95% CI 0.91–1.28, p = 0.38), and the hazard ratio for death among those who had total mastectomy without irradiation as compared with those who underwent radical mastectomy was 1.03 (95% CI 0.87–1.23, p = 0.72). Among women with clinically positive nodes, the hazard ratio for death among those who underwent total mastectomy and radiation as compared with those who underwent radical mastectomy was 1.06 (95% CI 0.89–1.27, p = 0.49). These findings validate earlier results showing no advantage from radical mastectomy.1 Although differences in outcome of a few percentage points cannot be excluded with this sample size, these findings do not demonstrate a clinically significant survival advantage from removing occult positive nodes at the time of initial surgery or from the addition of locoregional radiation to total mastectomy. In patients presenting with clinically node-negative disease, pathologic evaluation of the radical mastectomy specimen revealed that 40% of cases were pathologically node positive. Because of randomization, one would assume that 40% of the patients randomized to total mastectomy with or without radiation were also node positive at presentation and had disease that was either radiated or not treated at all. However, the axillary failure rate in the TM-alone arm was only 19%, indicating that microscopic, occult disease in the axilla may not always progress into clinically overt disease. Furthermore, the similar overall (OS) survival between the 3 arms suggests that an axillary node dissection for the clinically-negative axilla is largely prophylactic, and that outcome is not likely to be significantly compromised by deferring the dissection until there is clinical evidence of disease in the axilla.
Perhaps more importantly, the initial results from the B-04 trial helped pave the way for the conduct of the NSABP B-06 trial evaluating even less radical surgical procedures for the treatment of early-stage breast cancer.
The NSABP B-06 trial compared lumpectomy and axillary node dissection with or without breast irradiation with modified radical mastectomy in patients with tumors 4 cm or less in greatest diameter. Between 1976 and 1984, 2163 patients were randomized to modified radical mastectomy, lumpectomy plus axillary node dissection plus breast irradiation, or lumpectomy and axillary node dissection. Similar to prior reports,2,7 the last update from that trial continues to demonstrate the value of lumpectomy and breast irradiation as the preferred treatment for the majority of patients with invasive operable breast cancer. After 20 years of follow-up,8 there continue to be no significant differences in OS, disease-free survival (DFS), or distant disease-free survival between the total mastectomy group and the groups treated with lumpectomy with or without breast irradiation. The hazard ratio (HR) for death with lumpectomy alone compared to total mastectomy was 1.05 (95% CI 0.90–1.23, p = 0.51). The hazard ratio (HR) for death with lumpectomy plus breast irradiation compared to total mastectomy, was 0.97 (95% CI 0.83–1.14, p = 0.74). Among lumpectomy-treated women with tumor-free margins, the hazard ratio for death among those who received postoperative breast irradiation compared to those who did not was 0.91 (95% CI 0.77–1.06, p = 0.23). Radiation therapy was associated with a marginally significant decrease in deaths due to breast cancer. However, this decrease was partially offset by an increase in deaths from other causes.
Despite lack of significant differences in overall survival, significant differences in local control of the disease were observed among the 3 arms of the B-06 trial. In-breast recurrence occurred in 39.2% of the patients randomized to lumpectomy alone compared to 14.3% in those randomized to lumpectomy plus breast irradiation. Chest wall recurrence was observed in 10.2% of the patients in the mastectomy group. Nearly three-quarters of the local recurrences in the lumpectomy-alone group occurred within the first 5 years after surgery, compared to the lumpectomy and breast irradiation group, in which only 40% of recurrences occurred within the first 5 years.
The NSABP B-06 trial, along with other trials including those conducted by the Milan group evaluating quadrantectomy,4,5 were instrumental in establishing breast-conserving surgery plus breast irradiation as the preferred method for the local treatment of patients with operable breast cancer.9 These results were further confirmed in a meta-analysis of all the randomized clinical trials.10
One of the commonly asked questions following disclosure of the results from the NSABP B-06 and the Milan trials was whether all patients with invasive breast cancer undergoing lumpectomy need postoperative radiotherapy. It was hypothesized that patients with small tumors (≤1 cm) could potentially be spared from radiotherapy because they have lower rates of local recurrence. It was further argued at the time (1990 NIH Consensus Development Conference) that patients with negative nodes and tumors upto 1 cm may not even need adjuvant systemic therapy because of their good prognosis. Since the NSABP B-06 trial (as well as the NSABP B-14 trial, described later in the chapter) did not include a sufficient number of patients with tumors upto 1 cm, to adequately address the radiotherapy or the tamoxifen questions respectively, the NSABP designed protocol B-21 to address these questions in a randomized prospective trial (Fig. 42-1). The primary aims of the trial were to examine whether tamoxifen was as effective as breast irradiation in preventing in-breast recurrence, and whether the addition of tamoxifen to breast irradiation was superior to breast irradiation alone in terms of local and systemic control of the disease. Women with node-negative invasive breast cancer upto 1 cm in diameter treated with lumpectomy and axillary dissection were randomized to tamoxifen alone, breast irradiation plus tamoxifen for 5 years, or breast irradiation plus placebo for 5 years. A total of 1009 patients were randomized (tamoxifen: n = 336; radiation and placebo: n = 336; radiation and tamoxifen: n = 337). Although the B-21 trial did not reach the originally required sample size of 2000 patients, recently published results11 demonstrated that radiation and placebo resulted in a 49% lower hazard rate of in-breast recurrence compared to tamoxifen alone; radiation and tamoxifen resulted in a 63% lower rate of in-breast recurrence compared to radiation and placebo. When compared with tamoxifen alone, radiation and tamoxifen resulted in an 81% reduction in hazard rate of in-breast recurrence. Cumulative incidence of in-breast recurrence through 8 years was 16.5% with tamoxifen alone, 9.3% with breast irradiation and placebo, and 2.8% with breast irradiation and tamoxifen (Fig. 42-1). Breast irradiation reduced in-breast recurrence below the level achieved with tamoxifen alone, regardless of estrogen receptor (ER) status. Distant treatment failures were infrequent and not significantly different among the 3 groups (p = 0.28). When tamoxifen-treated women were compared with those who received breast irradiation and placebo, there was a significant reduction in contralateral breast cancer (hazard ratio 0.45, p = 0.039). Overall survival was not different between the 3 groups (93%, 94%, and 93%, respectively; p = 0.93). Thus, this trial demonstrated that in node-negative patients with small invasive tumors treated with lumpectomy, tamoxifen was not as effective as breast irradiation in controlling the disease in the breast. It further showed that the combination of tamoxifen and breast irradiation resulted in better local control of the disease in the breast than either modality alone.
Figure 42-1
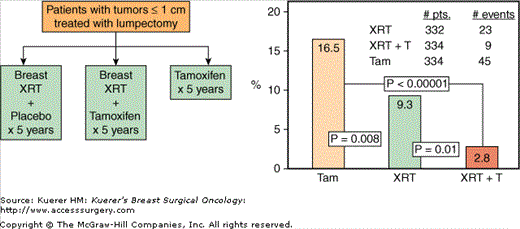
A. Schema of the NSABP B-21 trial evaluating the role of tamoxifen, breast radiotherapy (XRT), and a combination of the 2 in patients with invasive breast cancer ≤ 1 cm in size treated with lumpectomy. B. Comparison of the 8-year cumulative incidence of ipsilateral breast tumor recurrence (IBTR) rates between the 3 groups. (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326.)
Despite several decades of clinical investigations on prognostic factors for recurrence in patients with operable breast cancer, the status of the axillary lymph nodes has remained the single most important independent prognostic factor. None of the newer imaging modalities (such as magnetic resonance imaging [MRI], positron emission tomographic [PET] scan, or sestamibi scan) has been shown to be as accurate as pathologic examination of the axillary nodes in predicting nodal status. Thus, surgical excision of the axillary nodes still represents the gold standard for staging the axilla.
As mentioned above, initial randomized trials evaluating less radical procedures for the surgical treatment of operable breast cancer have demonstrated that elective axillary dissection did not impact survival when compared to delayed axillary dissection (if and when the axillary lymph nodes became clinically palpable).1,6 Thus, for several years it has been accepted that elective axillary dissection is mainly performed for staging purposes, to aid in the selection of appropriate adjuvant therapy, and for local control of the disease in the axilla. Recent randomized trials, however, have demonstrated a small but statistically significant survival advantage by adding locoregional radiation postmastectomy, in patients with positive axillary nodes who receive adjuvant chemotherapy.12,13 As an alternative to radiotherapy, axillary dissection provides excellent local control of the disease in the axilla in patients with positive axillary nodes. Whether axillary dissection is performed merely for staging purposes or whether it is performed for a possible small therapeutic benefit in patients with positive nodes, the axillary nodes are found to be histologically negative in the majority of patients with operable breast cancer (about 75%) at the time of surgery. These patients do not derive any therapeutic benefit from the axillary dissection but could experience significant postoperative morbidity. The desire to avoid an axillary dissection in these node-negative patients without losing the prognostic information derived from knowledge of the nodal status has led to the development of lymphatic mapping and sentinel node biopsy.
During the past decade, sentinel node biopsy (SNB) has become the procedure of choice for staging the axilla in patients with operable breast cancer. The feasibility and accuracy of this technique has been demonstrated in multiple single-institution,14-20 multicenter,21-25 and randomized clinical trials,26-31 as well as in a meta-analysis that included 69 studies and over 10,000 patients.32 Although outcome results from large randomized trials comparing SNB with axillary node dissection are not available yet, results from a smaller randomized trial—including 516 patients with a median follow-up of 46 months—have shown no differences in axillary recurrence or overall survival between patients receiving SNB alone and those receiving a completion axillary dissection.31 However, due to the small number of patients included in this trial, the confidence intervals around the estimates of outcome are wide.
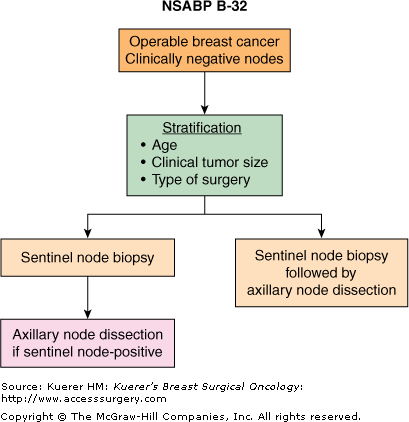
The NSABP B-32 trial was developed to definitively compare SNB alone to SNB followed by an axillary dissection in clinically node-negative patients with operable breast cancer.33 Patients were randomized to undergo SNB with mandatory concomitant axillary dissection (group 1) or SNB followed by axillary dissection only if the sentinel node revealed metastatic disease (group 2) (Fig. 42-2). Patients in the SNB-alone group who were found to have a positive sentinel node were required to also undergo full axillary dissection. In addition to its main objective, which is to compare the 2 procedures in terms of outcome, the study addresses a number of important biological and clinical questions such as the prognostic significance of immunohistochemically detected tumor cells in the SN when a routine H&E stain is negative, and quality-of-life factors related to lymphedema and arm function. Between 1999 and 2004, 5611 patients were randomized. Although the trial is not mature yet for survival analysis, technical reports on the performance of the procedure have shown an identification rate of 97% and a false-negative rate of 9.7%. The proportion of patients with metastatic sentinel nodes was 26% for both arms of the study. In nearly two-thirds of the cases, the sentinel nodes were the only sites of metastatic adenopathy.33-35
Whole-breast irradiation (delivered in 5-days-per-week fractions over a 5/6-week period) represents the standard approach for patients with early-stage breast cancer treated with lumpectomy. However, most in-breast recurrences occur near the lumpectomy scar, and in-breast events that occur in separate quadrants are frequently new primary breast tumors that develop with longer follow-up. The incidence of these second primaries is not necessarily affected by the delivery of postlumpectomy breast irradiation. These patterns of local failure following lumpectomy have provided the rationale for exploring the use of accelerated partial-breast irradiation (APBI) techniques. Possible advantages of APBI include the feasibility of completing adjuvant breast irradiation in a shorter time interval (usually within a week) and the potential for repeating breast-sparing surgery when an in-breast event occurs outside of the irradiated lumpectomy bed. In addition, given the short duration of the regimen, breast irradiation can precede the administration of adjuvant chemotherapy in patients who require both. APBI may also increase the use of breast-conserving therapy in women who may be reluctant to have whole-breast irradiation. However, before APBI can be considered in place of whole-breast irradiation, large-scale randomized clinical trials must demonstrate comparable clinical safety and efficacy between APBI and whole-breast irradiation.
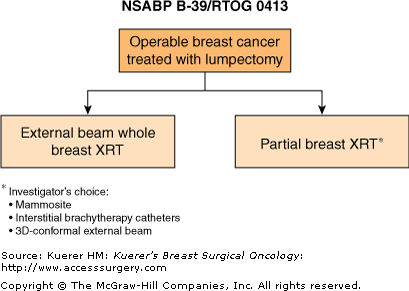
One such trial, currently conducted in North America by the NSABP and the Radiation Treatment Oncology Group (RTOG) is NSABP B-39/RTOG 0413 (Fig. 42-3).33,36 This trial will randomize a total of 4300 women to receive either conventional whole-breast irradiation or APBI. Eligible patients must have stage 0 ductal carcinoma in situ (DCIS) or stage I or II invasive adenocarcinoma of the breast, 3 cm or less in size, with no evidence of metastatic disease. For patients with positive axillary nodes, eligibility is restricted to those with 1 to 3 positive nodes. Women must have undergone a lumpectomy with histologically free margins. Participating institutions have the option of delivering APBI via high-dose multi-catheter brachytherapy, high-dose single-catheter balloon brachytherapy, or 3-dimensional conformal external beam irradiation. The primary end point of the trial is in-breast recurrence. The study will also compare the overall survival, recurrence-free survival, and distant disease-free survival between women receiving APBI and those receiving whole-breast irradiation. It will also examine quality-of-life issues related to cosmesis, fatigue, treatment-related symptoms, and perceived convenience of care. The trial opened to accrual in March 2005 with a target sample size of 3000 patients. In December 2006, accrual was closed to the low-risk cohorts of patients (women over the age of 50 with node-negative and ER-positive invasive tumors and women over the age of 50 with DCIS). In addition, the total sample size was increased to 4300. As of August 2009, more than 3550 patients have been entered and randomized.
Trial Evaluating “Adjuvant” Chemotherapy in Patients Who Develop In-Breast Recurrence or Other Locoregional Recurrence
The increasing use of lumpectomy as the preferred surgical procedure for operable breast cancer has resulted in a corresponding increase in the number of in-breast recurrences despite a reduction in the rate of recurrence secondary to improvements in surgery, radiotherapy, and the expanded use of adjuvant systemic therapy. As a result, there continues to be a need to further improve our understanding of the biological and clinical significance of in-breast recurrence and, more importantly, to further explore new therapeutic strategies for this group of patients. The need for the latter has become increasingly important since several reports published during the past decade have identified in-breast recurrence as an independent predictor of distant metastases. Following in-breast recurrence, the 5-year overall survival rate is in the range of 45% to 79%. In a recent report from 5 NSABP node-positive trials, the overall annual mortality rate for the first 5 years after an in-breast recurrence was about 14%, again underscoring the poor outcome of these patients.37
Adequate surgical management, the addition of locoregional radiotherapy, and the widespread use of adjuvant systemic therapy, have also resulted in a substantial decrease in the rate of locoregional recurrence (chest wall recurrence after mastectomy or regional lymph node recurrence irrespective of surgical procedure). However, approximately 10% to 20% of breast cancer patients with stages I to IIIa will have a locoregional recurrence alone, or as a component of distant failure within 10 years of undergoing mastectomy. Development of locoregional recurrence as the first event has long been associated with poor prognosis, with the majority of patients developing distant disease in a relatively short period of time. For node-positive patients, the average annual mortality rate in the first 5 years following locoregional recurrence ranges from 21.8% to 29.0%.37
Thus, it is quite evident that patients who develop an in-breast recurrence after lumpectomy, chest wall recurrence after mastectomy, or regional nodal recurrence irrespective of surgical procedure, are at significant risk for subsequent development of distant metastases and death from breast cancer. This information provides justification for focusing our research efforts to identify ways to improve the outcome of these patients. There is scant information regarding the worth of systemic therapy at the time of local or regional recurrence,38 and most of the available information relates to the use of hormonal therapy.39 To date, there have been no reported prospective randomized trials evaluating the worth of chemotherapy at the time of in-breast or other locoregional recurrence. As a result, there is widespread variability in the use of systemic chemotherapy at the time of locoregional recurrence. Data from 5 NSABP studies in node-positive patients indicate that “adjuvant” chemotherapy use at the time of in-breast recurrence varied between 18% and 40% of the cases.37 One of the reasons for not evaluating additional “adjuvant” chemotherapy at the time of in-breast or other locoregional recurrence is that, until the development of taxanes, there were no candidate non–cross-resistant regimens with promising activity that could be tested in this setting. However, the demonstration of significant antitumor activity with taxanes in patients resistant to anthracyclines and alkylating agents, as well as the recent successful results with sequential anthracycline-taxane regimens in the adjuvant and neoadjuvant settings,40-43 provided the opportunity to test this question in a randomized clinical trial.
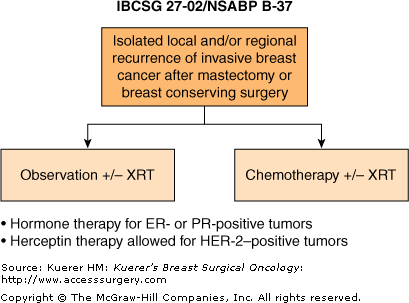
One such currently ongoing trial is the IBCSG (International Breast Cancer Study Group) 27-02/NSABP B-37 trial, which randomizes patients with resected in-breast recurrence or other locoregional recurrence to “adjuvant” chemotherapy or observation, in addition to hormonal therapy for hormone-receptor-positive tumors (Fig. 42-4).33 Eligible patients are randomized within 6 weeks of resection and treated within 4 weeks after randomization. Locoregional treatment consists of surgery and radiotherapy whenever possible. Radiotherapy is mandatory for patients who have not received prior adjuvant radiotherapy. Patients allocated to chemotherapy will preferentially receive combination chemotherapy with 2 or more drugs for at least 3 cycles. All patients with hormone-receptor-positive (ER+ and/or PgR+) recurrences will receive hormonal treatment. Trastuzumab therapy is permissible for HER-2/neu (HER-2)–positive patients, but this should be declared prior to randomization. A total of 977 patients will need to be randomized in this currently ongoing trial, and the primary end point is disease-free survival. Over 130 patients have been randomized to date.
As randomized trials demonstrated the value of breast-conserving surgery in patients with invasive breast cancer, the obvious question arose relative to the value of this procedure in patients with noninvasive disease. In addition, the introduction and widespread use of mammography has contributed to a dramatic increase in the incidence of small, localized, nonpalpable DCIS, an entity with excellent prognosis after local therapy alone. According to SEER data, DCIS accounts for approximately 20% of all breast cancers diagnosed in the United States, with age-adjusted incidence rates of 30 cases per 100,000 women, and approximately 47,000 cases detected each year.44 Following the results of the B-06 and other trials described above, in the early 1980s there was a paradox in the surgical treatment of early-stage breast cancer, with invasive disease being treated progressively more with lumpectomy, whereas mastectomy remained the recommended surgical treatment for noninvasive disease. Thus, it became imperative at the time to test the value of breast conservation in patients with DCIS. The NSABP was the first group to conduct such a prospective randomized trial.
The NSABP B-17 trial compared lumpectomy alone to lumpectomy plus breast irradiation in 818 patients with localized DCIS (Fig. 42-5).3,45-47 A mastectomy control group was not included, given the acceptance of lumpectomy based on the results of the B-06 trial, as well as the excellent prognosis of patients with localized DCIS. Recently updated results from the B-17 trial, after 12 years of follow-up,47 continue to indicate—as previously reported3,45—that radiotherapy significantly decreases the rate of invasive and noninvasive ipsilateral breast tumor recurrence. The cumulative incidence of noninvasive ipsilateral breast cancer recurrence as a first event was significantly reduced with breast irradiation from 14.6% to 8.0% (p = 0.001) (Fig. 42-5). More importantly, the cumulative incidence of invasive ipsilateral recurrence was also significantly reduced from 16.8% to 7.7% (p = 0.00001) (Fig. 42-5). No difference in overall survival has been observed between the 2 groups (86% vs 87%, p = 0.80), but over two-thirds of the deaths in this trial were not breast-cancer related. In a subset of 623 out of 814 evaluable patients from this trial, pathologic features were analyzed relative to their prognostic significance for ipsilateral breast cancer recurrence.46 Only the presence of moderate/marked comedo necrosis was a statistically significant independent predictor of in-breast recurrence in both treatment groups. Moreover, breast irradiation markedly reduced the annual hazard rates for in-breast recurrence in all subgroups of patients.
Figure 42-5
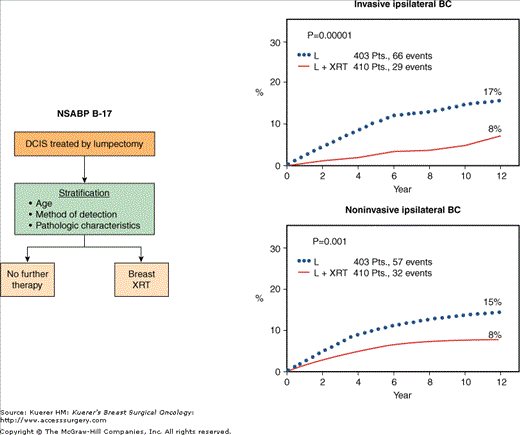
A. Schema of the NSABP B-17 trial comparing lumpectomy alone to lumpectomy plus breast irradiation (XRT) in patients with localized ductal carcinoma in situ (DCIS). B. Comparison of the 12-year cumulative incidence of invasive and noninvasive breast cancer recurrence between patients receiving lumpectomy alone (L) and those receiving lumpectomy plus breast radiotherapy (L+XRT). (BC, breast cancer.) (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326.Adapted from Fisher B, Land SR, Mamounas EP, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400-418.)
Following completion of the B-17 trial, another randomized trial was conducted by the NSABP in patients with DCIS to evaluate the role of tamoxifen after lumpectomy and breast irradiation (NSABP B-24).47,48 At that time, a large body of scientific evidence had accumulated demonstrating benefit from adjuvant tamoxifen in patients with resected early-stage invasive breast cancer.49 In these patients, tamoxifen not only reduced the risk for systemic recurrence but also had a significant impact in reducing the rate of in-breast recurrence following lumpectomy and breast irradiation.50,51 More importantly, tamoxifen reduced the incidence of second primary breast cancers in the contralateral breast by about 40%.49-55 The latter observation—along with preclinical evidence that tamoxifen inhibits both the initiation and promotion of tumors in experimental animals56,57—made tamoxifen an attractive agent for DCIS patients treated with lumpectomy and breast irradiation, for possibly reducing the rate of ipsilateral and contralateral invasive breast cancer events. Between 1991 and 1994, 1804 women with DCIS treated with lumpectomy were randomized to receive postoperative radiotherapy and either tamoxifen 20 mg daily for 5 years or placebo daily for 5 years (Fig. 42-6). In contrast to the B-17 trial, where lumpectomy margins were required to be free of DCIS for eligibility, in the B-24 trial patients were eligible whether the lumpectomy margins were free, involved, or unknown. As a result, about 75% of the patients in the B-24 trial had free lumpectomy margins, about 16% had involved margins, and in 10% the margins were unknown. Updated results after 7 years of follow-up continue to demonstrate, as previously reported,48 that the addition of tamoxifen significantly improved DFS from 77.1% to 83.0% (p = 0.002). This improvement was mainly the result of a reduction in the incidence of invasive and noninvasive breast cancer events in the ipsilateral as well as in the contralateral breast. The cumulative incidence of all ipsilateral and contralateral breast cancer events was reduced by 39%, from 16.0 % in the placebo group to 10.0% in the tamoxifen group (p = 0.0003).47 Tamoxifen reduced the rate of all invasive breast cancer events by 45% (p = 0.0009) and the rate of noninvasive breast cancer events by 27% (p = 0.11). When the effect of tamoxifen was examined according to the location of the first event, the cumulative incidence of ipsilateral breast cancers was reduced by 31% (11.1% with tamoxifen vs 7.7% with placebo, p = 0.02) and the cumulative incidence of contralateral breast cancers was reduced by 47% (4.9% vs 2.3%, p = 0.010) (Fig. 42-6). Several patient and tumor characteristics were found to increase the rate of in-breast recurrence, such as age under 50, involved/unknown lumpectomy margins, presence of comedo necrosis, and DCIS presentation with clinical findings. The effect of tamoxifen in reducing in-breast recurrence was evident irrespective of age, margin status, or presence/absence of comedo necrosis. However, for women with clinically apparent DCIS at study entry, in-breast recurrence rates were similar between the tamoxifen and placebo groups although the number of patients in that category was small.
Figure 42-6
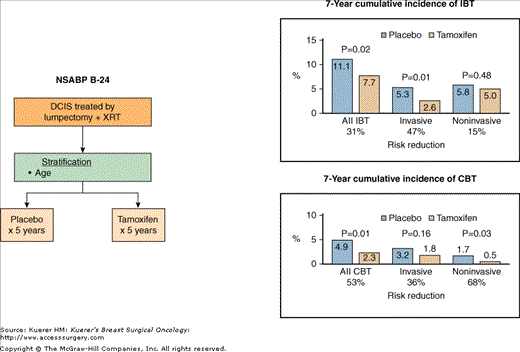
A. Schema of the NSABP B-24 trial comparing placebo with tamoxifen in patients with DCIS treated with lumpectomy plus breast radiotherapy (XRT). B. Comparison of the 7-year cumulative incidence of ipsilateral breast cancer events (IBT) and contralateral breast cancer events (CBT) between patients receiving placebo and those receiving tamoxifen. (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326.Adapted from Fisher B, Land SR, Mamounas EP, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400-418.)
The results from the B-24 trial indicate a significant benefit from tamoxifen in patients with DCIS. When these results are viewed together with those demonstrating benefit from tamoxifen in women with prior invasive breast cancer49-55 and in women with atypical hyperplasia and lobular carcinoma in situ (LCIS),58 they support the use of tamoxifen in the entire spectrum of breast neoplasia. However, one outstanding question following disclosure of the B-24 results was whether the observed benefit from tamoxifen was limited to subsets of DCIS patients. Given the strong association between ER expression and tamoxifen benefit in patients with invasive breast cancer, presence of a similar association might also be expected in patients with DCIS. In 2002, Allred and associates presented data from the NSABP B-24 trial on tamoxifen benefit according to ER status of the primary DCIS tumor.59 Of the 1804 patients participating in the trial, information on ER status was available in 628 patients (327 placebo, 301 tamoxifen). Seventy-seven percent of the patients had ER-positive tumors, and in these patients, the effectiveness of tamoxifen was clear (relative risk [RR] for all breast cancer events, 0.41; p = 0.0002). Significant reductions in breast cancer events were seen in both the ipsilateral and the contralateral breast. In patients with ER-negative tumors, little benefit was observed (RR for all breast cancer events, 0.80; p = 0.51), but the total number of events in this cohort was too small to rule out a small, clinically meaningful benefit. However, when these results are taken together with those evaluating the effect of tamoxifen in patients with invasive breast cancer and negative estrogen receptors, they are consistent with the observation that tamoxifen has no appreciable benefit in reducing rates of recurrence or rates of contralateral breast cancer in patients with ER-negative tumors. Furthermore, these results suggest that routine assessment of ER status should now be performed also in patients with DCIS to determine their candidacy for tamoxifen therapy.
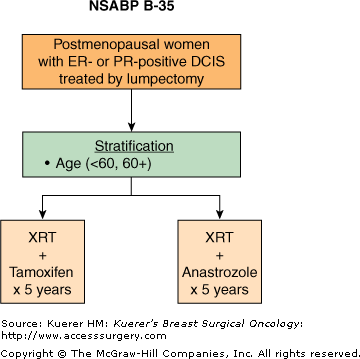
In the 1990s significant enthusiasm developed with the demonstration of considerable activity and favorable toxicity profile with third-generation aromatase inhibitors in patients with hormone-responsive, advanced breast cancer.60-66 As a result, several clinical trials have evaluated aromatase inhibitors as adjuvant therapy in patients with early-stage breast cancer. The first one to report results was the ATAC trial, which randomized more than 9000 early-stage breast cancer patients to receive anastrozole, tamoxifen, or the combination of anastrozole and tamoxifen, as adjuvant hormonal therapy.67 Besides yielding provocative results regarding the activity of anastrozole in the adjuvant treatment of ER-positive postmenopausal breast cancer, this trial was the first to demonstrate that the risk of contralateral new primary breast cancers was significantly reduced with anastrozole when compared to tamoxifen (odds ratio 0.42, p = 0.007).68 In addition, anastrozole demonstrated a more favorable side-effect profile when compared to tamoxifen (reduction in endometrial cancer, vaginal bleeding and discharge, cerebrovascular events, venous thromboembolic events, and hot flashes).67 On the other hand, when compared to tamoxifen, anastrozole resulted in significantly more myoskeletal disorders and fractures, which may have significant implications for the long-term use of this drug in patients with DCIS. The efficacy and safety results from the ATAC trial were eventually confirmed in other similar large trials evaluating aromatase inhibitors in the adjuvant setting and provided ample rationale for the evaluation of aromatase inhibitors in patients with DCIS. These findings prompted the design and conduct of NSABP B-35, a phase III trial that randomized postmenopausal patients with localized, ER-positive and/or PgR-positive DCIS treated with lumpectomy plus breast irradiation to tamoxifen versus anastrozole for 5 years (Fig. 42-7). The primary aim of the trial is to evaluate the effectiveness of anastrozole compared to tamoxifen in preventing the subsequent occurrence of breast cancer (local, regional and distant recurrences, and contralateral breast cancer). In addition, the trial aims to ascertain the effects of anastrozole on patient symptoms and quality of life as compared to tamoxifen. This protocol has completed its accrual goal after accruing over 3100 patients and is currently awaiting maturation of follow-up.
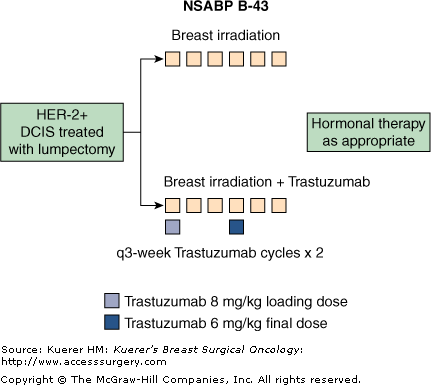
DCIS is more likely to overexpress HER-2 than is invasive cancer, and HER-2 overexpression is often associated with large-cell, comedo variety DCIS and more aggressive behavior.69,70 Trastuzumab is more likely to be effective in DCIS because it intervenes earlier in the carcinogenic pathway where there are fewer alternative escapes, and HER-2–positive DCIS is more likely than invasive breast cancer to depend on a single pathway. About 45% of patients with ER-negative DCIS and about 20% of patients with ER-positive DCIS overexpress HER-2.71 Preclinical data demonstrate that radiation enhances the anti- tumor effect of trastuzumab in tumors that overexpress the HER-2 oncogene.72 Based on this rationale, along with the safety and efficacy data with adjuvant trastuzumab in several randomized trials (“Studies Evaluating Biologic Targeted Therapies in the Adjuvant Setting” section (p. 487)),73,74 the NSABP developed protocol B-43, a phase III randomized trial to evaluate trastuzumab for patients with DCIS whose tumors overexpress HER2. Patients with HER-2–overexpressing DCIS treated with lumpectomy will be randomized to breast irradiation versus breast irradiation plus 2 doses of trastuzumab, starting on day 1 of breast irradiation and repeated once more 3 weeks later (Fig. 42-8). The primary end point of the study is the development of any breast cancer event. Secondary end points include in-breast recurrence and development of contralateral breast cancer. The NSABP B-43 trial opened 11/10/2008, with a goal of 2000 participants. As of September 2009, accrual is at 5.95%, with 119 patients.
During the last 20 years the NSABP has also played a significant role in conducting randomized trials that lead to the acceptance of adjuvant chemotherapy and adjuvant hormonal therapy for the treatment of breast cancer patients with negative axillary nodes. Beginning in the early 1980s several important trials evaluated in a stepwise fashion the worth of chemotherapy and that of hormonal therapy in patients with ER-negative tumors (Fig. 42-9) as well as in those with ER-positive tumors (Fig. 42-10).
Figure 42-9
NSABP adjuvant trials in node-negative breast cancer patients with ER-negative tumors evaluating, in a stepwise fashion, combination chemotherapy regimens, tamoxifen, and celecoxib. (*B-36 also includes patients with ER- or PR-positive tumors.) (AC, doxorubicin/cyclophosphamide; CEL, celecoxib; CMF, cyclophosphamide/methotrexate/5-fluorouracil; FEC, 5-fluorouracil/ epirubicin/cyclophosphamide; MF, methotrexate/5-fluorouracil with leucovorin rescue; TAM, tamoxifen.)
Figure 42-10
NSABP adjuvant trials in node-negative breast cancer patients with ER-positive tumors evaluating, in a stepwise fashion, tamoxifen, chemotherapy plus tamoxifen, exemestane, and letrozole (*B-33 and B-42 also include patients with node-positive tumors.) (AI, aromatase inhibitor; CMFT, cyclophosphamide/methotrexate/5-fluorouracil/tamoxifen; EXE, exemestane; LET, letrozole; MFT, methotrexate/5-fluorouracil/tamoxifen; TAM, tamoxifen.)
The NSABP B-13 trial randomized patients with negative nodes and negative estrogen receptors to surgery alone or surgery followed by 12 months of adjuvant chemotherapy with methotrexate and sequentially administered 5-fluorouracil (5-Fu) (M-F) followed by leucovorin (Figs. 42-9 and 42-11). Findings through 14 years of follow-up75 demonstrate that the improvements in disease-free and overall survival from M-F, previously reported after 576 and 877 years, have persisted (p < 0.0001 for the former and p = 0.02 for the latter) (Fig. 42-11). A statistically significant DFS was evident both for women less than 50 years of age (p = 0.005) as well as in those 50 years or more (p = 0.001). A statistically significant benefit in terms of survival was evident only in women 50 years of age or more (p = 0.02). For women less than 50 years of age, there was a nonsignificant trend toward improvement in overall survival with M-F (p = 0.3). However, there was no statistically significant evidence of an interaction between treatment group and age relative to overall survival (p = 0.34).
Figure 42-11
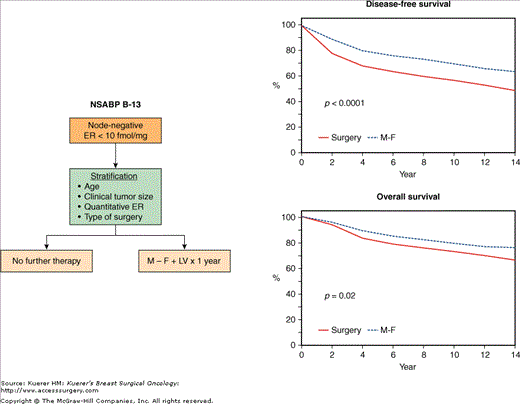
A. Schema of the NSABP B-13 trial comparing surgery alone with surgery followed by adjuvant methotrexate and 5-fluorouracil with leucovorin rescue (M – F + LV) in node-negative patients with estrogen receptor (ER)-negative tumors. B. Comparison of the 14-year disease-free and overall survival rates between the 2 groups. (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326. Adapted from Fisher B, Jeong JH, Dignam J, et al. Findings from recent National Surgical Adjuvant Breast and Bowel Project studies of stage I breast cancer. JNCI Monographs. 2001;30:62-66. Oxford University Press.)
Following completion of the NSABP B-13, a subsequent trial in the same patient population (NSABP B-19) attempted to determine whether the alkylating agent cyclophosphamide contributed additional benefit when administered with methotrexate and 5-FU (CMF regimen as developed by the Milan Group) (Figs. 42-9 and 42-12). Over a 6-month period, patients received either 6 courses of M-F or 6 courses of CMF. A total of 1095 patients were randomized. Through 8 years of follow-up,75 just as first reported after 5 years,77 the results continue to demonstrate a statistically significant disease-free and overall survival advantage with CMF over M-F (p = 0.003 and p = 0.03, respectively) (Fig. 42-12). Those advantages were most evident in women aged upto 50 years (p = 0.0004 and p = 0.007, respectively). In women aged more than 50 years, there was a small but nonsignificant advantage in both disease-free survival (p = 0.2) and overall survival (p = 0.8). As was also observed in the B-13 study, there was no evidence of a statistically significant interaction between treatment and age group relative to disease-free survival (p = 0.22) and overall survival (p = 0.08).
Figure 42-12
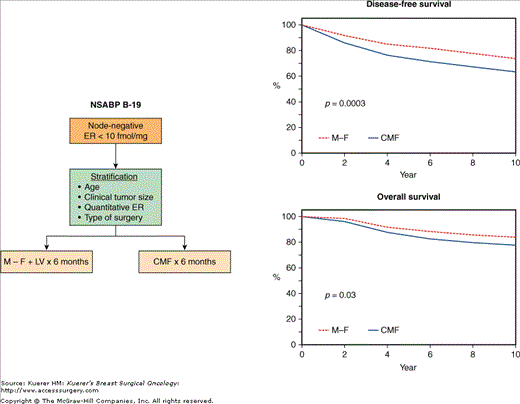
A. Schema of the NSABP B-19 trial comparing adjuvant methotrexate/5-fluorouracil/leucovorin (M − F + LV) with adjuvant cyclophosphamide/methotrexate/5-fluorouracil (CMF) in node-negative patients with estrogen receptor (ER)-negative tumors. B. Comparison of the 8-year disease-free and overall survival rates between the 2 groups. (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326. Adapted from Fisher B, Jeong JH, Dignam J, et al.Findings from recent National Surgical Adjuvant Breast and Bowel Project studies of stage I breast cancer. JNCI Monographs. 2001;30:62-66. Oxford University Press.).
Following completion of the B-19 trial, the NSABP initiated protocol B-23, which attempted to determine whether tamoxifen has a role in patients with ER-negative tumors. In this study, patients with negative nodes and ER-negative tumors were randomized to 4 cycles of adjuvant doxorubicin/cyclophosphamide (AC) or 6 cycles of adjuvant CMF with or without tamoxifen (Fig. 42-9). The rationale for evaluating tamoxifen in patients with ER-negative tumors came both from preclinical and clinical observations. Results from several preclinical studies had demonstrated that tamoxifen acts not only through a blockade of the ER pathway but also by modulating the production of growth factors such as TGF-α and TGF-β, by increasing the levels of sex-hormone-binding globulin in the serum, by increasing natural killer cell counts, and by decreasing insulin-like growth factor. In addition, at the time of initiation of this study there was considerable clinical information suggesting that tamoxifen prolongs disease-free survival and survival irrespective of receptor status although the benefit in ER-negative tumors was of lesser magnitude.78-81 Since that time, an increasing body of evidence has demonstrated that tamoxifen confers no significant advantage in patients with ER-negative tumors. The results of the B-23 trial confirmed these latter observations and demonstrated no significant prolongation in DFS or OS with the addition of tamoxifen to chemotherapy (DFS: CMF, 83%; CMF + tamoxifen, 83%; AC, 83%; AC + tamoxifen, 82%; OS: CMF, 89%; CMF + tamoxifen, 89%; AC, 90%; AC + tamoxifen, 91%).82 The results of the B-23 trial also confirmed (in the node-negative setting) the previous observation from the NSABP B-15 trial (in node-positive patients) that 4 cycles of AC were equivalent to 6 cycles of CMF in terms of disease-free and overall survival prolongation. One interesting observation in this trial was that tamoxifen did not confer a significant reduction in the incidence of contralateral breast cancer as it has been shown in patients with ER-positive tumors and negative nodes.50,75 Explanation for this discrepancy was recently provided by a retrospective review of several NSABP trials, which found that there was significant concordance between the ER status of the primary breast cancer and that of contralateral breast cancer.83 Thus, in about 80% of patients who initially present with an ER-negative primary and who develop contralateral breast cancer, the contralateral breast tumor is also ER-negative, making the potential chemopreventative effect of tamoxifen negligible.
One of the approaches utilized in order to improve the outcomes of CMF-treated node-negative breast cancer patients is the incorporation of doxorubicin into the chemotherapy regimen. As noted above, protocol B-23 demonstrated the equivalence between 6 cycles of CMF and 4 cycles of AC. However, several other trials have shown that 6 cycles of CAF/CEF/FAC or FEC are superior to 6 cycles of CMF.84-87 These results suggest that cycle duration may be important in optimizing results with anthracycline-based regimens. Results from adjuvant trials evaluating 6 cycles of a dose-intense epirubicin-based regimen also suggested that the epirubicin-based regimens may be more effective than doxorubicin-based regimens.88 The NIH Consensus Conference on Adjuvant Therapy of Breast Cancer held in November 2000 stressed the need for trials to address the issues of cycle duration with anthracycline-based chemotherapy.89
Moreover, an evolving body of preclinical and clinical literature had suggested that induction of cyclooxygenase 2 (COX-2) expression and the resultant increase in prostaglandins may contribute to the development of the malignant phenotype in breast cancer and other malignancies. Availability of well-tolerated, selective inhibitors of COX-2 provided an opportunity to evaluate COX-2 as a target for cancer treatment and prevention in women with node-negative breast cancer.
Thus, the NSABP B-36 trial (Fig. 42-9) was built on the experience from the previous NSABP trials, to evaluate chemotherapy in high-risk node-negative breast cancer patients. The trial randomizes women with node-negative breast cancer to either 4 cycles of AC or 6 cycles of FEC. The original design of the trial included a secondary randomization in a 2 × 2 factorial design to celecoxib versus placebo. However, the recent removal of rofecoxib from the market due to cardiovascular toxicities observed with COX-2 inhibitors resulted in modification of the B-36 trial and discontinuation of the randomization between celecoxib and placebo. The B-36 trial completed its accrual after accruing 2722 patients and is currently awaiting maturation of follow-up.
Parallel to the studies evaluating chemotherapy and tamoxifen in patients with node-negative, ER-negative breast cancer, the NSABP launched a series of trials that initially evaluated tamoxifen and the combination of tamoxifen plus chemotherapy in patients with node-negative, ER-positive disease and subsequently evaluated aromatase inhibitors as extended adjuvant therapy, either after 5 years of tamoxifen (B-33) or after 5 years of an aromatase inhibitor (B-42) in node-negative and node-positive patients with hormone-receptor-positive breast cancer (Fig. 42-10).
The NSABP B-14 trial randomized patients after surgery to 5 years of tamoxifen or 5 years of placebo (Figs. 42-10 and 42-13). Published results from this trial through 10 years of follow-up51 continue to demonstrate a statistically significant disease-free survival benefit from tamoxifen (69% vs 57%, p < 0.0001). In addition, a significant survival advantage was demonstrated in this update (80% vs 76%, p = 0.02). The disease-free and overall survival advantage with tamoxifen was evident both in women < 50 as well as in those ≥ 50 years of age. Tamoxifen therapy continued to demonstrate a significant reduction in the rate of contralateral breast cancer (4.0% vs 5.8%, p = 0.007).
The significant advantage for disease-free and overall survival with tamoxifen has now persisted through 14 years of follow-up (Fig. 42-13).75 In a most recent update90 with 15 years of follow-up, the benefit from tamoxifen continues to be evident irrespective of age, menopausal status, or tumor estrogen-receptor concentration (hazard ratio [HR] for recurrence-free survival 0.58, 95% CI 0.50–0.67, p < 0.0001; HR for overall survival 0.80, 95% CI 0.71–0.91, p = 0.0008).
Figure 42-13
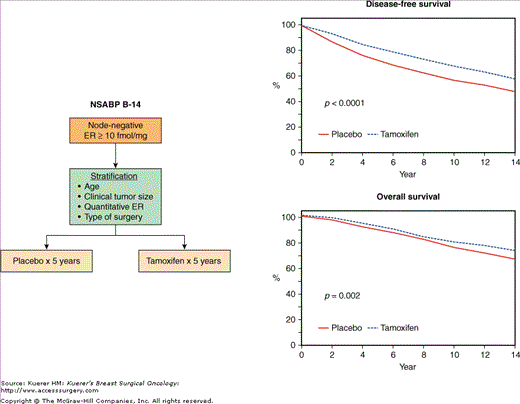
A. Schema of the NSABP B-14 trial comparing 5 years of placebo with 5 years of tamoxifen in node-negative, ER-positive patients. B. Comparison of 14-year disease-free and overall survival rates between the 2 groups. (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326. Adapted from Fisher B, Jeong JH, Dignam J, et al.Findings from recent National Surgical Adjuvant Breast and Bowel Project studies of stage I breast cancer. JNCI Monographs. 2001;30:62-66. Oxford University Press.)
One of the most common questions asked while the NSABP B-14 trial was being conducted related to the optimal duration of tamoxifen administration. By design, patients were to receive 5 years of tamoxifen or 5 years of placebo. To answer the question of optimal tamoxifen duration beyond 5 years, patients randomized to tamoxifen who were alive and recurrence-free following 5 years of treatment were asked to be rerandomized to 5 additional years of tamoxifen or 5 years of placebo.51 Originally reported results, through 4 years from rerandomization, demonstrated a significant disadvantage in disease-free survival (86% vs 92%, p = 0.003) and distant disease-free survival (90% vs 96%, p = 0.01) for patients who continued tamoxifen for more than 5 years versus those who discontinued the drug at 5 years.
Overall survival was 96% for those who discontinued tamoxifen compared with 94% for those who continued it (p = 0.08). Updated results, through 7 years from the time of rerandomization continue to demonstrate no additional benefit from the prolonged tamoxifen administration.91 In fact, a slight advantage continues to exist for patients who discontinued tamoxifen after 5 years relative to those who continued to receive it (disease-free survival, 82% vs 78%, p = 0.03; relapse-free survival, 94% vs 92%, p = 0.13; overall survival, 94% vs 91%, p = 0.07, respectively). The lack of benefit from additional tamoxifen therapy was independent of age or other characteristics.
An important observation from the B-14 trial was that through 10 years of follow-up of tamoxifen-treated patients with ER-positive, node-negative breast cancer, the disease-free survival (69%) and overall survival (80%) were not as good as originally thought for this group of patients, who are generally considered to have a favorable prognosis.51 These numbers further decreased after 14 years of follow-up,75 with disease-free survival being around 60% and overall survival around 75%. Although a small proportion of the events included in the disease-free and overall survival analyses are non–breast-cancer related, these results underscore the need for further improvement of the outcome of these patients. Subsequent to the B-14 trial, the NSABP conducted protocol B-20 that evaluated the worth of adding chemotherapy to tamoxifen in patients with negative nodes and positive ER (Fig. 42-14). Between 1988 and 1993, 2363 patients were randomized to receive either tamoxifen for 5 years, or tamoxifen plus 6 cycles of sequential methotrexate and 5-Fu followed by leucovorin (MFT) or tamoxifen plus 6 cycles of cyclophosphamide, methotrexate, and 5-Fu (CMFT). Through 5 years of follow-up, the combination of chemotherapy plus tamoxifen resulted in significantly improved disease-free survival over tamoxifen alone (90% for MFT vs 85% for tamoxifen, p = 0.01; 89% for CMFT vs 85% for tamoxifen, p = 0.001). A similar benefit was observed in overall survival (97% for MFT vs 94% for tamoxifen, p = 0.05; 96% for CMFT vs 94% for tamoxifen, p = 0.03).92 The reduction in recurrence and mortality was greatest in patients aged 49 years or less. All subgroups of patients evaluated in this study benefited from chemotherapy. The results from the B-20 study were updated with 8 years of follow-up and continue to demonstrate a significant improvement in disease-free and overall survival with the addition of chemotherapy to tamoxifen when compared to tamoxifen alone (84% vs 77%, p = 0.001, for disease-free survival; 92% vs 88% for overall survival, p = 0.018) (Fig. 42-14).75 The most recent update with 12 years of follow-up continues to demonstrate a significant improvement in disease-free survival and a borderline significant improvement in overall survival with the addition of chemotherapy.90
Figure 42-14
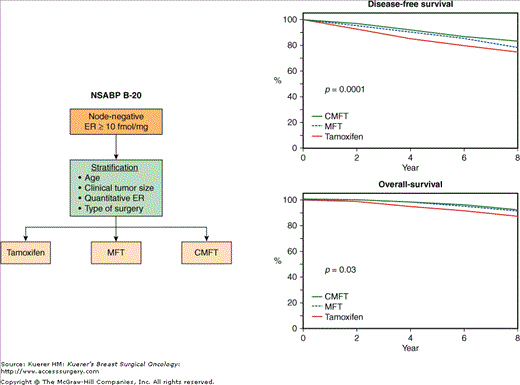
A. Schema of the NSABP B-20 trial comparing adjuvant tamoxifen alone with adjuvant tamoxifen plus methotrexate/5-fluorouracil plus leucovorin (MFT) and with tamoxifen plus cyclophosphamide/methotrexate/5-fluorouracil (CMFT) in node-negative patients with ER-positive tumors. B. Comparison of the 8-year disease-free and overall survival rates between the 3 groups of patients. (Reproduced with permission from Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309-326. Adapted from Fisher B, Jeong JH, Dignam J, et al.Findings from recent National Surgical Adjuvant Breast and Bowel Project studies of stage I breast cancer. JNCI Monographs. 2001;30:62-66. Oxford University Press.)
An important remaining question following the disclosure of the NSABP B-14 and NSABP B-20 trials was whether we can identify subgroups of these node-negative, ER-positive patients at low risk for recurrence when treated with tamoxifen alone, which can be spared chemotherapy administration. In the past few years, genomic profiling of the primary breast tumor has shown considerable promise toward that goal. Using archival paraffin block material from the NSABP B-14 and NSABP B-20 trials, Genomic Health, in collaboration with the NSABP, developed and validated a reverse transcriptase polymerase chain reaction (RT-PCR)-based 21-gene assay (also know as 21-gene recurrence score or OncotypeDX) that predicts outcome and benefit from hormonal therapy and chemotherapy in these patients (Fig. 42-15).93,94 This assay is currently commercially available for use in ER-positive, node-negative patients. According to the results of this assay, ER-positive, node-negative breast cancer patients with a low recurrence score have low risk of recurrence and receive little benefit from the addition of adjuvant chemotherapy to hormonal therapy. Those with a high recurrence score have a considerably high risk of recurrence and receive significant benefit from the addition of adjuvant chemotherapy to hormonal therapy and should be treated with both. A prospective randomized clinical trial (TAILORx) is currently being conducted to evaluate whether the addition of adjuvant chemotherapy to hormonal therapy is necessary for patients who have an intermediate recurrence score.
Figure 42-15
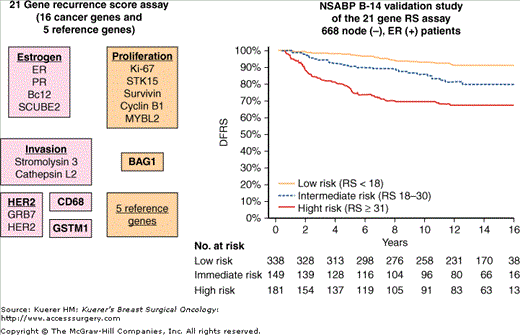
A. Gene panel for the 21-gene Recurrence Score Assay (OncotypeDX). B. Results of the NSABP B-14 validation study of the 21-gene Recurrence Score Assay. (DRFS, distant recurrence-free survival; ER, estrogen receptor; RS, recurrence score.) (Adapted with permission from Paik S, Shack S, Tang J, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817-2826. Copyright © 2004 Massachusetts Medical Society. All rights reserved.)
The results from the NSABP B-14 trial demonstrating no additional benefit from continuing tamoxifen therapy for longer than 5 years,91 as well as the appreciation of continued risk for recurrence in patients with hormone-receptor-positive breast cancer after 5 years of tamoxifen95 led several investigators to consider additional adjuvant hormonal therapy interventions following completion of tamoxifen therapy. During the past several years, abundant information became available demonstrating substantial antitumor activity with the use of aromatase inhibitors in patients with advanced breast cancer who suffered a recurrence during or after tamoxifen therapy. Thus, attempting to further reduce the risk of subsequent recurrence in patients who remain disease free after completion of adjuvant tamoxifen by administering aromatase inhibitors became an important clinical research question. Based on the above rationale, the NSABP developed protocol B-33, a randomized trial comparing exemestane with placebo in postmenopausal patients who complete 5 years of tamoxifen and are recurrence-free (Fig. 42-16).96 The primary aim of the trial was to determine whether exemestane will prolong DFS. Secondary aims were to determine whether exemestane will prolong OS, and to evaluate the effect of exemestane and that of tamoxifen withdrawal on fracture rate, bone mineral density, markers of bone turnover, levels of lipids and lipoproteins, and quality of life. Between May 2001 and October 2003, a total of 1598 women were randomized out of the 3000 required to complete the study. However, in October 2003, accrual to this study was terminated early per recommendation of the NSABP Data Monitoring Committee when results from a similar adjuvant trial (NCIC-CTG MA.17) became available demonstrating significant improvement with letrozole after 5 years of tamoxifen.97 As a result of these findings, all patients in the NSABP B-33 trial were unblinded and 5 years of exemestane was offered to both groups at no cost. Despite the premature closure of the trial (50% of the target accrual) and the ensuing crossover to exemestane (about 50% of patients), original assignment to exemestane versus placebo resulted in borderline improvement in disease-free survival and in a significant improvement in relapse-free survival of a similar magnitude to that seen in the NCIC MA.17 trial with letrozole.98
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree