Objectives
- 1.
Describe the organization of the male gonad, the testis, and the process of spermatogenesis, and discuss how this process is supported by Sertoli cells.
- 2.
Describe the steroidogenic pathway of Leydig cells that produces testosterone, the peripheral conversion of testosterone to estradiol-17β or dihydrotestosterone, and the actions of these steroids in men.
- 3.
Explain the regulation of testicular function by the hypothalamic-pituitary-testicular axis.
- 4.
Describe the role of the proximal male reproductive tract, especially the epididymis, in the further development of sperm.
- 5.
Explain the more distal segments of the male reproductive tract, including the accessory sex glands, in the context of emission and ejaculation.
- 6.
Describe the neurovascular events in the penis that are involved in erection.
- 7.
Explain the following pathologic conditions of the male reproductive system: Klinefelter syndrome and androgen insensitivity that is coupled to testicular feminization.
In men, the reproductive system has evolved for continuous, lifelong gametogenesis, coupled to occasional internal insemination with a high density of sperm (greater than 60 × 10 6 /mL in 3 to 5 mL of semen). This means that in adult men, the basic roles of gonadal hormones are as follows:
- •
Support of gametogenesis (spermatogenesis)
- •
Maintenance of the male reproductive tract and production of semen
- •
Maintenance of secondary sex characteristics and libido. There is no overall cyclicity of this activity in men.
Histophysiology of the Testis
A major difference between the testes and the ovaries is that the testes reside in the scrotum outside of the abdominopelvic cavity and are connected to the abdominopelvic male tract by the spermatic cord (see Fig. 8.2 in Chapter 8 ). Various mechanisms, including the cremasteric reflex, a venous countercurrent exchanger in the spermatic cord (pampiniform plexus), folding of scrotal skin, and sweat glands within the scrotal skin, cooperate to maintain a testicular temperature at about 35°C, which is crucial for sperm development. Failure of the testes to descend through the inguinal canal into the scrotum during development results in depressed spermatogenesis and an increased risk of testicular cancer.
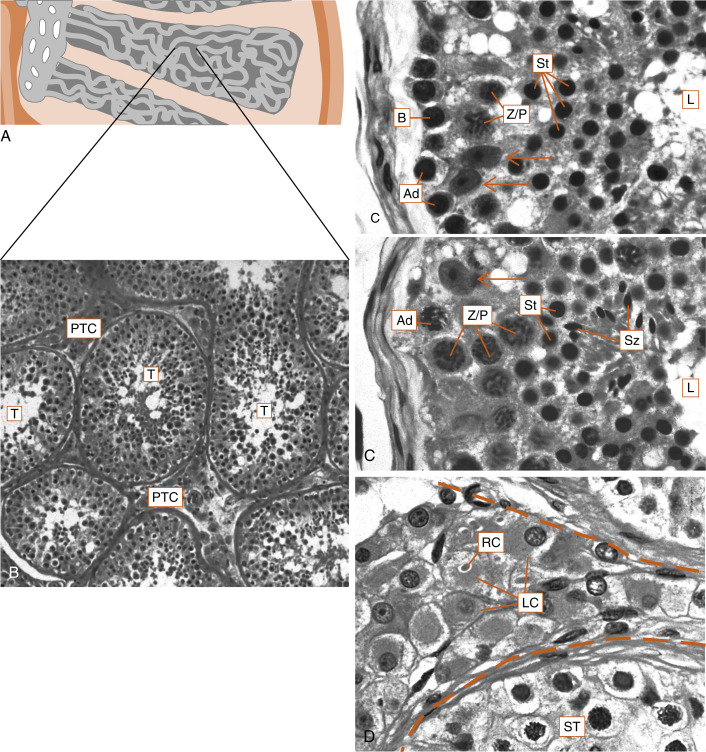
The human testis is covered by a connective tissue capsule and is divided into about 300 lobules by fibrous septa. Within each lobule are two to four loops of seminiferous tubules ( Fig. 9.1 ). Each loop empties into an anastomosing network of tubules called the rete testis . The rete testis is continuous with small ducts, the efferent ductules that lead the sperm out of the testis into the head of the epididymis on the superior pole of the testis (see Fig. 9.8 ). Once in the epididymis, the sperm pass from the head, to the body, to the tail of the epididymis and then to the vas (ductus) deferens . Spermatozoa are stored in the tail of the epididymis and the vas deferens for several months as viable sperm.
The presence of the seminiferous tubules in the lobules of the testis creates two compartments within each lobule: an intratubular compartment , which is composed of the seminiferous epithelium of the seminiferous tubule; and a peritubular compartment , which is composed of neurovascular elements, connective tissue cells, immune cells, and the interstitial cells of Leydig, whose main function is to produce testosterone ( Fig. 9.1D ).
The Intratubular Compartment
The seminiferous tubule is lined by a complex seminiferous epithelium ( Fig. 9.2 ) composed of two cell types:
- •
Sperm cells in various stages of spermatogenesis
- •
The Sertoli cell, which is a nurse cell in intimate contact with all sperm cells and which regulates many aspects of spermatogenesis
Developing Sperm Cells
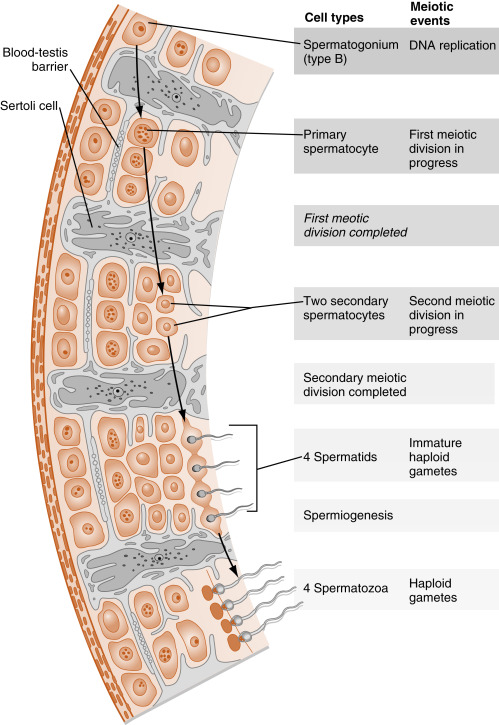
The entire developmental process by which spermatogonia give rise to spermatozoa is called spermatogenesis . Spermatogenesis begins at puberty and involves the processes of mitosis and meiosis ( Fig. 9.2 ). Stem spermatogonia (also called prespermatogonia) reside at the basal level of the seminiferous epithelium. Stem spermatogonia divide mitotically to generate daughter spermatogonia ( spermatocytogenesis ). These mitotic divisions are initially asymmetric, in that one daughter cell remains a stem spermatogonium (thereby undergoing self-renewal throughout life), whereas the second daughter cell will divide several times to amplify its population. After several mitotic divisions, the daughter spermatogonia complete S phase (DNA replication) and commit to meiotic division. Of note, these amplifying divisions are accompanied by incomplete cytokinesis, so all spermatogonia daughter cells and subsequent sperm cells (at different stages) remain interconnected by a cytoplasmic bridge. This configuration contributes to the synchrony of development of a clonal population of sperm cells.
Spermatogonia migrate apically away from the basal lamina as they enter the first meiotic prophase (see Fig. 9.2 ). At this time, they are called primary spermatocytes . During the first meiotic prophase, the hallmark processes of sexual reproduction involving synapsis, crossing-over , formation of chiasmata , and the first disjunction occur (see Chapter 8 ). Completion of the first meiotic division gives rise to secondary spermatocytes , which quickly (within 20 min) complete the second meiotic division.
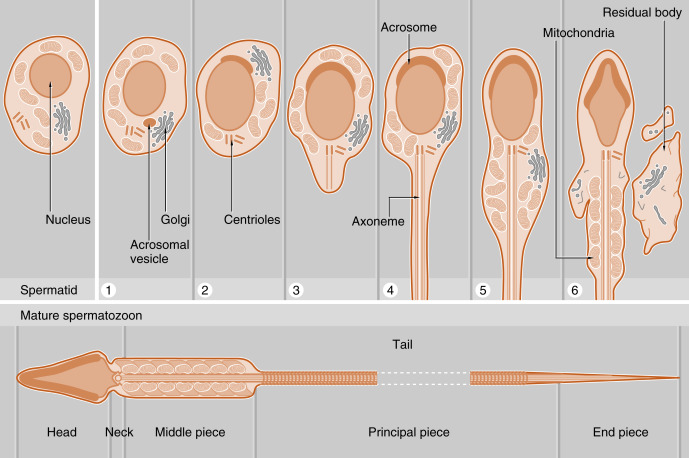
The initial products of meiosis are haploid spermatids , which reside apically within the seminiferous epithelium, close to the lumen of the seminiferous tubule (see Fig. 9.2 ). Spermatids are small, round cells with a round nucleus. Spermatids undergo a remarkable metamorphosis called spermiogenesis that results in a spermatozoon ( Fig. 9.3 ). The spermatozoon contains the following parts:
- 1.
A head . The head consists of two major components:
- a.
A condensed and streamlined nucleus . The chromatin of the nucleus is highly heterochromatic (i.e., condensed), and the nucleosomal histones are replaced by protamines . The DNA of a spermatozoan is transcriptionally silent.
- b.
An acrosomal vesicle . The acrosomal vesicle contains hydrolytic enzymes transported to it from the Golgi. These enzymes will play an important role in fertilization and the prevention of polyspermy (see Chapter 11 ). The acrosomal vesicle attaches to the forward pole of the nucleus and descends along the side of the nucleus so that it partially covers the nucleus.
- a.
- 2.
The neck . This contains two centrioles (proximal and distal). The proximal centriole attaches to the nucleus, and the distal centriole will generate a “9 + 2” configuration of microtubules that is called the axoneme .
- 3.
The tail (also called the flagellum ). The tail has a continuous axonemal core but is composed of structurally distinct regions called the middle piece, principal piece, and end piece. The middle piece is the thickest and contains a collar of mitochondria that will deliver adenosine triphosphate (ATP) for flagellar beating and motility. The outer circumference of the middle piece contains dense fibers. The principal piece and the end piece lack the mitochondrial sheath, and the end piece lacks the outer dense fibers.
The process of spermatogenesis takes about 72 days. A cohort of adjacent spermatogonia enters the process every 16 days, so the process is staggered along the length of a seminiferous tubule. Consequently, spermatogonia do not enter the process of spermatogenesis at the same time along the entire length of the tubule, or in synchrony with every other tubule (there are about 500 seminiferous tubules per testis). Because the seminiferous tubules within one testis total about 400 m in length, spermatozoa are continually being generated at many sites within the testis at any given time. Histologic examination of the seminiferous tubules reveals that there are specific associations, called spermatogenic stages , of sperm cells at any one point in time. In humans, there are six different stages that progress and repeat as a cycle at one point within the seminiferous tubule. This is referred to as the spermatogenic cycle . The stages are staggered spatially along the length of the seminiferous tubule, before repeating themselves. This spatial configuration of cycles is called a spermatogenic wave .
The final release of sperm, called spermiation , is an active process involving dissolution of adhesive junctions between Sertoli cells and spermatozoa. It is important to note that testicular spermatozoa after spermiation are not fully mature. Testicular spermatozoa are barely motile, and leave the seminiferous tubule passively within fluid produced by the Sertoli cells.
The Sertoli Cell
The Sertoli cell represents the true epithelial cell of the seminiferous epithelium and extends from the basal lamina to the lumen ( Box 9.1 ; see Fig. 9.2 ). Sertoli cells surround sperm cells, providing structural support within the epithelium, and form adherens-type junctions and gap junctions with all stages of sperm cells (see Fig. 9.2 ). Through the formation and breakdown of these junctions, Sertoli cells guide sperm cells toward the lumen as they advance to later stages in spermatogenesis. Accordingly, major secretory products of Sertoli cells include proteases and protease inhibitors. Spermiation requires the final breakdown of Sertoli cell–sperm cell junctions.
Supportive (“Nursing”)
- •
Maintaining, breaking, and reforming multiple junctions with developing sperm
- •
Maintaining blood-testis barrier
- •
Phagocytosis
- •
Transfer of nutrients and other substances from blood to developing sperm cells
- •
Expression of paracrine factors and receptors for sperm-derived paracrine factors
Exocrine
- •
Production of fluid to move immobile sperm out of testis toward epididymis
- •
Production of androgen-binding protein
- •
Determination of release of spermatozoa (spermiation) from seminiferous tubule
Endocrine
- •
Expression of androgen receptor and follicle-stimulating hormone receptor
- •
Production of Müllerian-inhibiting substance, also called anti-Müllerian hormone
- •
Aromatization of testosterone to estradiol-17β (this has local effect, not strictly endocrine)
Another important structural feature of Sertoli cells is the formation of tight junctions among adjacent Sertoli cells (see Fig. 9.2 ). These occluding junctions divide the seminiferous epithelium into a basal compartment , containing the spermatogonia and early-stage primary spermatocytes, and an adluminal (i.e., toward the lumen) compartment , containing later-stage primary spermatocytes and all subsequent stages of sperm cells. Because early primary spermatocytes move apically from the basal compartment to the adluminal one, the tight junctions need to be disassembled and reassembled. These tight junctions form the physical basis for the blood-testis barrier , which creates a specialized, immunologically safe microenvironment for developing sperm. By blocking paracellular diffusion, the tight junctions restrict movement of substances between the blood and the developing germ cells through a trans-Sertoli cell transport pathway and in this manner allow the Sertoli cell to control nutrient availability to germ cells. Accordingly, Sertoli cells also have the responsibility for providing nutrients to this environment, such as transferrin, iron, and lactate. For example, spermatogonia and released spermatozoa use fructose and glucose for energy. However, sperm undergoing meiosis cannot efficiently use glucose as an energy source. Sertoli cells acquire glucose by the GLUT1 transporter, metabolize it to lactate, and transfer it to developing sperm, which express a sperm-specific lactate transporter. This process is dependent on hormonal stimulation (follicle-stimulating hormone [FSH] and testosterone; see later in the text) but also appears to be optimized by local sperm cell–generated paracrine factors.
Thus healthy Sertoli cell function is essential for sperm cell viability and development. In this respect, it should be noted that spermatogenesis is absolutely dependent on testosterone produced by peritubular Leydig cells (see later), yet it is the Sertoli cells that express the androgen receptor , not the developing sperm cells. Similarly, the pituitary hormone FSH is also required for maximal sperm production, and again, it is the Sertoli cell that expresses the FSH receptor , not the developing sperm. Thus these hormones support spermatogenesis indirectly through stimulation of Sertoli cell function.
Sertoli cells have multiple additional functions. Sertoli cells express the enzyme CYP19 ( also called aromatase ), which converts Leydig cell–derived testosterone to the potent estrogen, estradiol-17β (see later in the text). This local production of estrogen may enhance spermatogenesis in humans. Sertoli cells also produce androgen-binding protein (ABP) . ABP is encoded by the same gene as for sex hormone–binding globulin (SHBG; Chapter 1 ; see later in the text) but has different carbohydrate groups and is specifically expressed intratesticularly. ABP maintains a high androgen level within the adluminal compartment, the lumina of the seminiferous tubules, and the proximal part of the male reproductive tract. Sertoli cells also produce a large amount of fluid. This fluid provides an appropriate bathing medium for the sperm and assists in moving the immotile spermatozoa from the seminiferous tubule into the epididymis. Sertoli cells perform an important phagocytic function . This allows Sertoli cells to engulf residual bodies , which represent cytoplasm that is shed by spermatozoa during spermiogenesis, as well as dead sperm cells.
Finally, the Sertoli cell has an important endocrine role. During development, Sertoli cells produce anti-Müllerian hormone (AMH) , also called Müllerian-inhibiting substance (MIS) , which induces regression of the embryonic Müllerian duct that is programmed to give rise to the female reproductive tract (see Chapter 8 ). The Sertoli cells also produce the hormone inhibin . Inhibin is a heterodimer protein hormone related to the transforming growth factor-β (TGF-β) family. FSH stimulates inhibin production, which then exerts negative feedback on gonadotropes to inhibit FSH production. Thus inhibin keeps FSH levels within a specific range (see later in the text).
The Peritubular Compartment
The peritubular compartment (see Fig. 9.1 ) contains the primary endocrine cell of the testis, the Leydig cell . This compartment also contains common cell types of loose connective tissue and an extremely rich peritubular capillary network that must provide nutrients to the seminiferous tubules (by way of Sertoli cells) while conveying testosterone away from the testes to the peripheral circulation.
The Leydig Cell
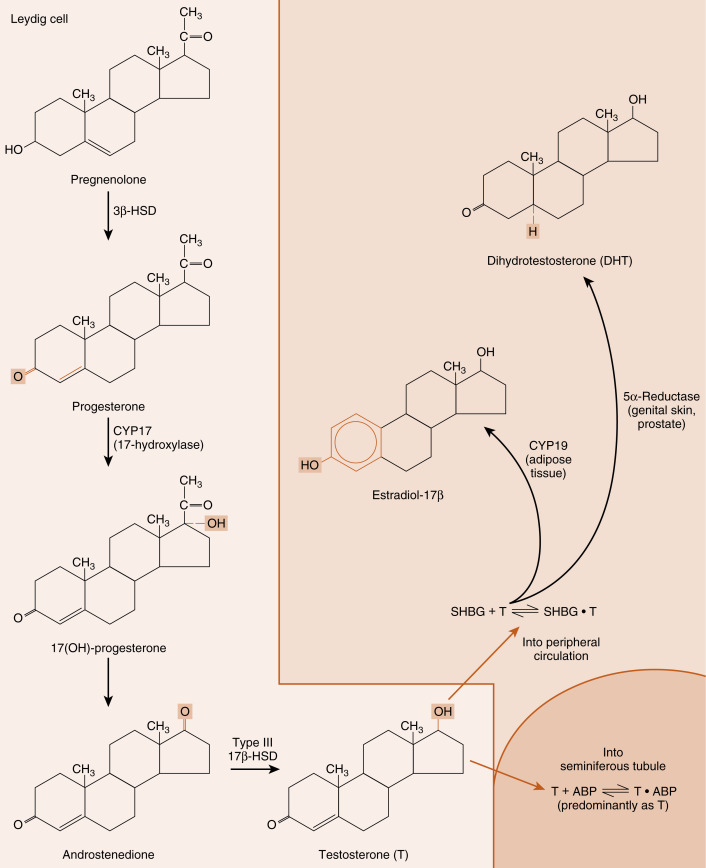
Leydig cells are steroidogenic stromal cells. These cells synthesize cholesterol de novo, as well as acquiring it through low-density lipoprotein (LDL) receptors and, to a lesser extent, high-density lipoprotein (HDL) receptors (the HDL receptor is also called scavenger receptor-B1 [SR-B1] ), and store cholesterol as cholesterol esters, as described for adrenocortical cells (see Chapter 7 ). Free cholesterol is generated by a cholesterol hormone-sensitive lipase (HSL) and transferred to the outer mitochondrial membrane, and then to the inner mitochondrial membrane in a steroidogenic acute regulatory protein (StAR) -dependent manner (refer to Fig. 7.6 in Chapter 7 ). As in all steroidogenic cells, cholesterol is converted to pregnenolone by CYP11A1 . Pregnenolone is then processed to progesterone, 17α-hydroxyprogesterone, and androstenedione by 3β-hydroxysteroid dehydrogenase type 2 (3β-HSD2) and CYP17 ( Fig. 9.4 ). Recall from Chapter 7 that CYP17 is a bifunctional enzyme, with a 17-hydroxylase activity and a 17, 20-lyase activity. CYP17 displays a robust level of both activities in the Leydig cell. In this respect, the Leydig cell is similar to the zona reticularis cell, except that it expresses a higher level of 3β-HSD, so that the Δ4 pathway is ultimately favored. Another major difference is that the Leydig cell expresses a Leydig cell–specific isoform of 17β-hydroxysteroid dehydrogenase (17β-HSD3) , which converts androstenedione to testosterone (see Fig. 9.4 ). Mutation of this specific gene in men results in a form of disorders of sexual development (DSD ; see later in the text).