Objectives
- 1.
Describe the anatomy and histology of the ovary and the development of the ovarian follicle.
- 2.
Describe the steroidogenic pathways in the ovarian follicle and the functions of the ovarian steroids, estradiol-17β and progesterone.
- 3.
Diagram the hypothalamus-pituitary-ovarian axis in the context of the monthly menstrual cycle.
- 4.
Explain the changes in the physiology of the female reproductive tract throughout the menstrual cycle.
- 5.
Describe the anatomy and function of the female external genitalia during the female sexual response.
- 6.
Explain pathophysiologic conditions of the female reproductive system, including Turner syndrome and polycystic ovarian syndrome.
The physiology of pregnancy and the development and functions of the placenta and mammary glands are discussed in Chapter 11 .
The female reproductive system is composed of the gonads, called ovaries , and the female reproductive tract. The mammary glands (breasts) are also part of the female reproductive system. Like the male gonads, the ovaries perform an endocrine function and a gametogenic function. The endocrine function is regulated within a hypothalamic-pituitary-ovarian axis, and ovarian hormones are absolutely necessary for the health and normal function of the female tract. The female reproductive system differs from the male system in several important general aspects ( Box 10.1 ).
| Male Reproductive System | Female Reproductive System |
|---|---|
| Gonads (testes) reside outside of abdominal cavity, in scrotum | Gonads (ovaries) reside within abdominal cavity |
| Gonad is continuous with reproductive tract | Gonad is not continuous with reproductive tract |
| Release of gametes (sperm) from gonads is continuous | Release of gamete (egg) from gonads occurs once per month |
| Gametic reserve is replenished throughout life | Gametic reserve is finite and exhausted by menopause |
| Testosterone exerts negative feedback on secretion of pituitary luteinizing hormone (LH) and follicle-stimulating hormone (FSH) | Estrogen exerts both negative and positive feedback on secretion of pituitary LH and FSH |
| Male tract serves only male gamete transport and maturation and delivery | Female tract serves male and female gamete transport and maturation, fertilization, placentation, and gestation |
| Activity of male tract does not show rhythm | Activity of female tract is based on the monthly menstrual cycle, or on the length of a pregnancy (normally about 9 months) |
| Testosterone is always the primary gonadal steroid | Estrogen is the primary gonadal steroid in the first half of the monthly cycle, and progesterone in the second half |
| The male reproductive system does not prepare for newborn | The female reproductive system prepares for newborn with breast development and milk production and is involved in breastfeeding of the newborn |
Anatomy and Histology of the Ovary
The ovary is located within a fold of peritoneum called the broad ligament, usually close to the lateral wall of the pelvic cavity ( Fig. 10.1 ). The ovary extends into the peritoneal cavity, and ovulated eggs briefly reside within the peritoneal cavity before they are captured by the oviducts. Nerves and blood vessels enter and exit the ovary at both its lateral and medial poles.
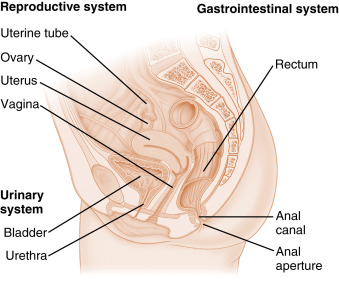
The ovary can be roughly divided into an outer cortex and an inner medulla ( Fig. 10.2 ). The neurovascular elements run into the medulla of the ovary. The cortex of the ovary is composed of a densely cellular stroma. Within this stroma reside the ovarian follicles , which contain a primary oocyte surrounded by follicle cells (see later in the text). The cortex is covered by a connective tissue capsule, called the tunica albuginea , and a layer of simple epithelium, called ovarian surface epithelial cells . There are no ducts emerging from the ovary to convey its gametes to the reproductive tract. Thus the process of ovulation involves an inflammatory event that erodes the wall of the ovary and follicle. After ovulation, the ovarian surface epithelial cells rapidly divide to repair the wall. It is this highly mitogenic population of cells, the ovarian surface epithelial cells, which gives rise to more than 80% of cases of ovarian cancer.
Growth, Development, and Function of the Ovarian Follicle
The ovarian follicle is the functional unit of the ovary, performing both gametogenic and endocrine functions. A histologic section of the ovary from a premenopausal cycling woman contains follicular structures at many different points of their development. The life history of a follicle can be divided into the following stages:
- 1.
Resting primordial follicle
- 2.
Growing preantral (primary and secondary) follicle
- 3.
Growing antral (tertiary) follicle
- 4.
Dominant (preovulatory, graafian) follicle
- 5.
Dominant follicle within the periovulatory period
- 6.
Corpus luteum (of menstruation or of pregnancy)
- 7.
Atretic follicles
In this section, follicular biology is discussed in terms of the following elements:
- •
Growth and structure of the follicle
- •
State of the gamete
- •
Endocrine function of the follicle cells
Resting Primordial Follicle
Growth and Structure
Resting primordial follicles represent the earliest and simplest follicular structure in the ovary. Primordial follicles appear during midgestation through the interaction of gametes and somatic cells. The approximately 7 million oogonia enter the process of meiosis, thereby becoming primary oocytes (see Chapter 8 ). The primary oocytes arrest in prophase of meiosis I.
During this time, the primary oocytes become surrounded by a simple epithelium of somatic follicle cells, thereby creating primordial follicles ( Fig. 10.3 ). The follicle cells (also called pregranulosa cells ) establish gap junctions with each other and with the oocyte. The follicle cells themselves represent a true avascular epithelium, surrounded by a basal lamina. As in Sertoli cell–sperm interactions, the granulosa cells remain intimately attached to the oocyte throughout its development. Granulosa cells provide nutrients such as amino acids, nucleic acids, and pyruvate to support oocyte maturation.

Primordial follicles represent the ovarian reserve of follicles ( Fig. 10.4 ). This is reduced from a starting number of about 7 million to fewer than 300,000 follicles at reproductive maturity. Of these, a woman will ovulate about 450 between menarche (first menstrual cycle) and menopause (cessation of menstrual cycles). At menopause, fewer than 1000 primordial follicles are left in the ovary. Primordial follicles are lost primarily from death due to follicular atresia . A small subset of primordial follicles, however, will enter follicular growth in waves. Because the ovarian follicular reserve represents a fixed, finite number, the rate at which resting primordial follicles die or begin to develop will determine the reproductive life span of a woman.
Determination of the age at which a woman will reach menopause has a strong genetic component but also is influenced by environmental factors. For example, cigarette smoking significantly depletes the ovarian reserve. An overly rapid rate of atresia or development also depletes the reserve, giving rise to premature ovarian failure, defined as entering menopause before the age of 40 years. Premature ovarian failure can also be caused by severe infections or tumors of the pelvis, by chemotherapy and radiation, and by endocrine factors that disrupt the hypothalamus-pituitary-ovarian axis.

The rate at which resting primordial follicles enter the growth process appears to be independent of pituitary gonadotropins. There is evidence in mice that follicle cells stimulate oocyte growth through paracrine factors. Reciprocal regulation of granulosa cell growth by the oocyte also probably occurs. Additional evidence indicates that factors from growing follicles provide restraint on the development of too many primordial follicles. One such factor appears to be anti-Müllerian hormone (AMH) . AMH-knockout mice deplete their ovarian reserve more rapidly than do wild-type mice, as a result of a high rate of follicular development. In summary, whether a resting follicle enters the early growth phase is dependent primarily on intraovarian paracrine factors that are produced by both the follicle cells and oocytes.
The Gamete
As mentioned previously, the gamete in primordial follicles is derived from oogonia that have entered the first meiotic division and are now called primary oocytes . These primary oocytes progress through most of prophase of the first meiotic division (termed prophase I ) over a 2-week period and then arrest in the diplotene stage. This stage is characterized by decondensation of chromatin, which supports transcription needed for oocyte maturation. Meiotic arrest at this stage, which may last up to 50 years, appears to be due to “maturational incompetence,” or the lack of necessary cell cycle proteins to support the completion of meiosis. The nucleus of the oocyte, called the germinal vesicle , remains intact at this stage.
Endocrine Function
Although primordial follicles release paracrine factors, they do not produce ovarian steroid hormones.
Growing Preantral Follicles
Growth and Structure
One of the first visible signs of follicle growth is the appearance of cuboidal granulosa cells surrounding the oocyte. At this point, the follicle is referred to as a primary follicle (see Fig. 10.3 ). While granulosa cells proliferate, they form a multilayered (i.e., stratified) epithelium around the oocyte. At this stage, the follicle is referred to as a secondary follicle (see Fig. 10.3 ). Before the formation of a fluid-filled antral cavity, primary and secondary follicles are referred to as preantral . Once a secondary follicle acquires three to six layers of granulosa cells, it secretes paracrine factors that induce nearby stromal cells to differentiate into epithelioid thecal cells. Thecal cells form a flattened layer of cells around the follicle. Once a thecal layer forms, the follicle is referred to as a mature preantral follicle (see Fig. 10.3 ). In humans, it takes several months for a primary follicle to reach the mature preantral stage.
Follicular development is associated with an inward movement of the follicle from the outer cortex to the inner cortex, closer to the vasculature of the ovarian medulla. Follicles release angiogenic factors that induce the development of one or two arterioles, which generate a vascular wreath around the follicle.
The Gamete
During the preantral stage, the oocyte begins to grow and produce cellular and secreted proteins. The oocyte initiates secretion of extracellular matrix glycoproteins, called ZP proteins 1 to 4, that form the zona pellucida (see Fig. 10.3 ). The zona pellucida ultimately increases to a thickness of 13 μm in humans and provides a species-specific binding site for sperm during fertilization (see Chapter 11 ). Of importance, granulosa cells and the oocyte project cellular extensions through the zona pellucida and maintain gap junctional contacts. The oocyte also continues to secrete paracrine factors that regulate follicle cell growth and differentiation.
Endocrine Function
The granulosa cells express the follicle-stimulating hormone (FSH) receptor during the preantral period but are dependent primarily on factors from the oocyte to grow. They do not produce ovarian hormones at this early stage of follicular development.
The newly acquired thecal cells are analogous to testicular Leydig cells (see Chapter 9 ), in that they reside outside of the epithelial nurse cells, express the luteinizing hormone (LH) receptor, and produce androgens. The main difference between Leydig cells and thecal cells is that thecal cells do not express high levels of a 17β-hydroxysteroid dehydrogenase (17β-HSD). Thus the major product of the thecal cells is androstenedione, as opposed to testosterone. Androstenedione production at this stage is absent or minimal.
Growing Antral Follicles
Growth and Structure
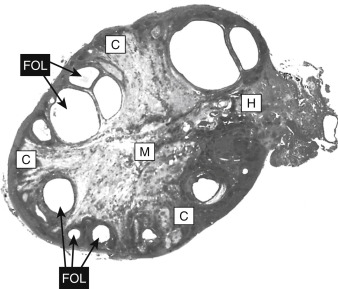
Mature preantral follicles develop into early antral follicles ( Fig. 10.5 ) over a period of about 25 days, growing from a diameter of approximately 0.1 to 0.2 mm. Once the granulosa epithelium increases to six or seven layers, fluid-filled spaces appear between cells and coalesce into the antrum. Over a period of about 45 days, this wave of small antral follicles will continue to grow to large, recruitable antral follicles that are 2 to 5 mm in diameter. This period of growth is characterized by about a 100-fold increase in granulosa cells (from about 10,000 to 1,000,000 cells). It is also characterized by swelling of the antral cavity, which increasingly divides the granulosa cells into two discrete populations ( Fig. 10.6 ; see Fig. 10.5 ):
- 1.
The mural granulosa cells (also called stratum granulosum ) are those that form the outer wall of the follicle. The basal layer is adhered to the basal lamina and in close proximity to the outer-lying thecal layers. Mural granulosa cells become highly steroidogenic and remain in the ovary after ovulation to differentiate into the corpus luteum.
- 2.
The cumulus cells are the inner cells that surround the oocyte (they are also referred to as the cumulus oophorus ). The layer of cumulus cells closest to the oocyte, the corona radiata , maintains gap and adhesion junctions with the oocyte. Cumulus cells are released from the ovary with the oocyte (collectively referred to as the cumulus-oocyte complex ) during the process of ovulation . Cumulus cells are crucial for the ability of the fimbriated end of the oviduct to “capture” and move the oocyte by a ciliary transport mechanism along the length of the oviduct to the site of fertilization.
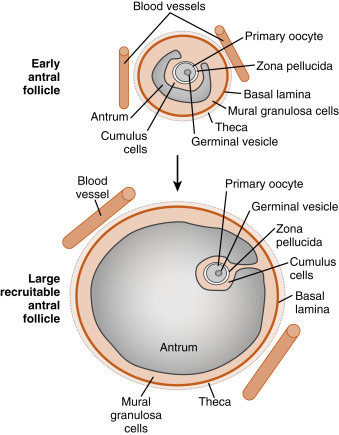
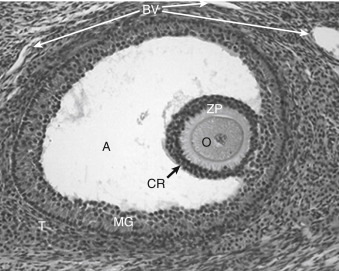
Early antral follicles are dependent on pituitary FSH for normal growth. Large antral follicles become highly dependent on pituitary FSH for their growth and sustained viability. As discussed later (under “Dominant Follicle”), 2- to 5-mm follicles are recruited to enter a rapid growth phase by a transient increase in FSH that occurs toward the end of a previous menstrual cycle.
The Gamete
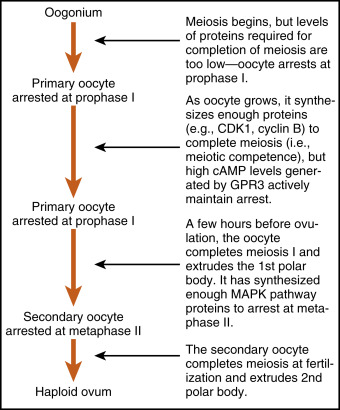
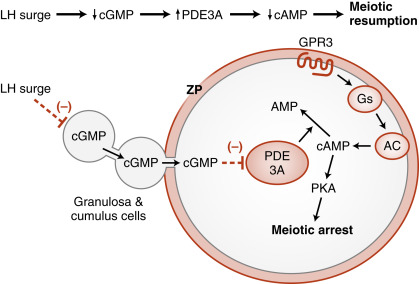
The oocyte grows rapidly in the early stages of antral follicles; growth then slows in larger follicles. During the antral stage, the oocyte synthesizes cell-cycle proteins, such as cyclin-dependent kinase-1 (CDK1) and cyclin B , and the oocyte becomes competent to complete meiosis I at ovulation. Thus in preantral follicles, the oocyte fails to complete meiosis I because of a dearth of specific meiosis-associated proteins (i.e., they are incompetent to complete meiosis I). Larger antral follicles, however, gain meiotic competence but still maintain meiotic arrest until the midcycle LH surge. Meiotic arrest is achieved in the meiotically competent, prophase-arrested oocyte by the maintenance of elevated cyclic adenosine monophosphate (cAMP) levels. The oocyte expresses a constitutively active (i.e., active without a ligand) G-protein–coupled receptor, called GPR3 , that maintains high cAMP levels ( Figs. 10.7 and 10.8 ). Through a cAMP-PKA phosphorylation cascade, the cyclin B–cyclin-dependent kinase, CDK1 , complex (also called maturation-promoting factor , or MPF ) is kept inactive. As discussed in Chapter 1 , intracellular cAMP levels are determined by the activity of adenylyl cyclase, which generates cAMP, and by the activity of phosphodiesterases (PDEs) , which metabolize cAMP to AMP. Cyclic guanosine monophosphate (cGMP) contributes to the maintenance-elevated cAMP levels in competent oocytes by inhibiting the oocyte-specific PDE, PDE3A . Cyclic GMP is made in the cumulus cells and mural granulosa cells and is transferred to the oocyte through gap junctions (see Fig. 10.8 ).
Endocrine Function
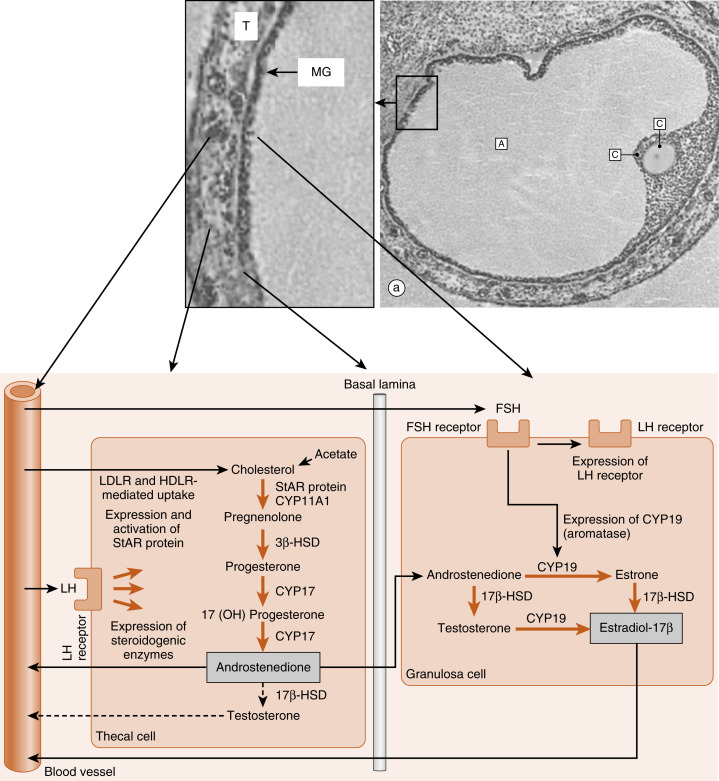
Thecal cells of large antral follicles produce significant amounts of androstenedione and, to a much lesser extent, testosterone ( Fig. 10.9 ). This is due to high expression of CYP17 with both 17-hydroxylase and 17,20-lyase activities ( Fig. 10.10A ). Androgens are converted to estradiol-17β by the mural granulosa cells ( see Fig. 10.9 ). At this stage, FSH stimulates proliferation of granulosa cells and induces the expression of CYP19-aromatase ( Fig. 10.10B ) required for estrogen synthesis. Additionally, the mural granulosa cells of the large antral follicles produce increasing amounts of inhibin B during the early follicular phase. Low levels of estrogen and inhibin exert a negative feedback effect on FSH secretion, thereby contributing to the selection of the follicle with the most FSH-responsive cells.
Dominant Follicle
Growth and Structure
At the end of a previous menstrual cycle, a crop of large (2- to 5-mm) antral follicles (see Fig. 10.4 ) is recruited to begin rapid, gonadotropin-dependent development. The total number of recruited follicles in both ovaries can be as high as 20 in a younger woman (<33 years of age), but rapidly declines at older ages. The number of recruited follicles is reduced to the ovulatory quota (which is one in humans) by the process of selection . While FSH levels decline, the rapidly growing follicles progressively undergo atresia, until one follicle is left. Generally, the largest follicle with the most FSH receptors of the recruited crop becomes the dominant follicle . Selection occurs during the early follicular phase. By midcycle, the dominant follicle becomes a large preovulatory follicle that is 20 mm in diameter and contains about 50 million granulosa cells by the midcycle gonadotropin surge.
The Gamete
The oocyte is competent to complete meiosis I but remains arrested in the dominant follicle through the mechanisms described earlier. Growth of the oocyte continues, but at a slower rate—the human oocyte reaches a diameter of about 140 μm by ovulation. The stalk by which cumulus cells are attached to the mural granulosa cells becomes increasingly attenuated.
Endocrine Function
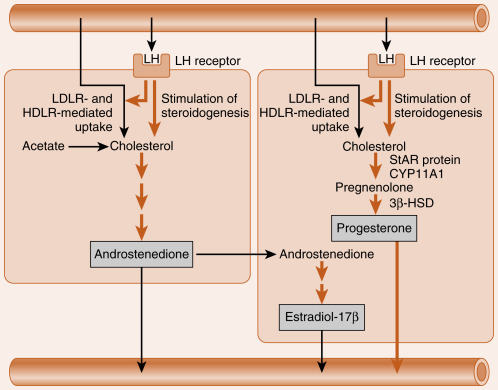
The newly selected follicle emerges for the first time during its development as a significant steroidogenic gland. Ovarian steroidogenesis requires both theca and granulosa cells (see Fig. 10.9 ). As discussed earlier, theca cells express LH receptors and produce androgens . Basal levels of LH stimulate the expression of steroidogenic enzymes, as well as the LDL receptor in the theca. Theca cells show robust expression of CYP11A1 (also called P-450 cholesterol side-chain cleavage ), 3β-hydroxysteroid dehydrogenase (3β-HSD), and CYP17 , with both 17-hydroxylase activity and 17,20-lyase activity . Androgens (primarily androstenedione but also some testosterone ) released from the theca can diffuse into the mural granulosa cells or can enter the vasculature surrounding the follicle.
The mural granulosa cells of the selected follicle have a high number of FSH receptors and are very sensitive to FSH signaling. FSH strongly upregulates CYP19 (aromatase) gene expression and activity (see Fig. 10.9 ). CYP19 converts androstenedione to the weak estrogen, estrone , and converts testosterone to the potent estrogen, estradiol-17β. Granulosa cells express activating isoforms of 17β-HSD , which ultimately drives steroidogenesis toward the production of estradiol-17β (see later in the text). FSH also induces the expression of inhibin B during the follicular phase.
Of importance, FSH also induces the expression of LH receptors in the mural granulosa cells during the second half of the follicular phase (see Fig. 10.9 ). Thus mural granulosa cells become responsive to both gonadotropins, allowing these cells to maintain high levels of CYP19 in the face of declining FSH levels. Acquisition of LH receptors also ensures that mural granulosa cells will respond to the LH surge (see later).
The Dominant Follicle During the Periovulatory Period
The periovulatory period can be defined as the time from the onset of the LH surge to the expulsion of the cumulus-oocyte complex out of the ovary (i.e., ovulation ). This process lasts 32 to 36 hours in women. Starting at the same time, and superimposed on the process of ovulation, is a change in the steroidogenic function of the theca and mural granulosa cells. This process is called luteinization and culminates in the formation of a corpus luteum that is capable of producing large amounts of progesterone , along with estrogen, within a few days after ovulation.
Growth and Structure
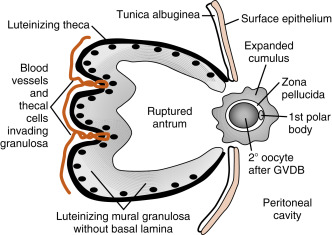
The LH surge induces dramatic structural changes in the dominant follicle that involve its rupture, ovulation of the cumulus-oocyte complex, and the biogenesis of a new structure called the corpus luteum from the remaining theca cells and mural granulosa cells. Major structural changes occur during this transition (see Fig. 10.10 ):
- 1.
Before ovulation, the large preovulatory follicle presses against the ovarian surface, generating a poorly vascularized bulge of the ovarian wall called the stigma . The LH surge induces the release of inflammatory cytokines and hydrolytic enzymes from the theca and granulosa cells. These secreted components lead to the breakdown of the follicle wall, tunica albuginea, and surface epithelium in the vicinity of the stigma. At the end of this process, the antral cavity becomes continuous with the peritoneal cavity.
- 2.
The stalklike attachment of the cumulus cells to the mural granulosa cells detaches, and the cumulus-oocyte complex becomes free-floating within the antral cavity. As an indirect response to the LH surge (i.e., in response to LH-dependent paracrine factors), the oocyte releases the transforming growth factor-β (TGF-β)–related factor, GDF9. GDF9 (see earlier in the text) stimulates the cumulus cells to secrete hyaluronic acid and other extracellular matrix components. These secreted components enlarge the entire cumulus-oocyte complex, a process called cumulus expansion . This enlarged cumulus-oocyte complex is more easily captured and transported by the oviduct. The expanded cumulus also makes the cumulus-oocyte complex easier for spermatozoa to find. The cumulus-oocyte complex is released through the ruptured stigma in a slow, gentle process, indicating that the follicular fluid in the antrum is not under increased pressure. The specific forces that lead to expulsion of the cumulus-oocyte complex are unknown.
- 3.
The basal lamina of the mural granulosa cells is enzymatically degraded, and blood vessels and outer-lying theca can push into the granulosa cells. Granulosa cells secrete angiogenic factors , such as vascular endothelial growth factor (VEGF) , angiopoietin-2 , and basic fibroblast growth factor (bFGF) , which significantly increase the blood supply to the new corpus luteum.
The Gamete
Before ovulation, the primary oocyte is competent to complete meiosis but is arrested in prophase I as a result of high cAMP levels (see Fig. 10.8 ). The LH surge induces the oocyte to progress to metaphase of meiosis II . The oocyte subsequently arrests at metaphase II until fertilization. LH receptors are only present on the mural granulosa cells. The LH surge induces a series of events that ultimately lead to a decrease in cGMP in the oocyte. This decrease in cGMP removes the inhibition on the cAMP phosphodiesterase, PDE3A, which now becomes active and degrades cAMP in the oocyte.
The decrease in cAMP and protein kinase A (PKA) activity in the oocyte ultimately leads to activation of MPF , composed of CDK1 and cyclin B . MPF drives nuclear events that complete meiosis I with the extrusion of the first polar body. The secondary oocyte (called an egg ) then arrests in metaphase of meiosis II . This is achieved by an increase in an activity called cytostatic factor (CSF). It is now known that CSF is composed of the kinase, c-Mos , its target mitogen-activated kinase kinase (MAPKK) , also called MEK1 (see Chapter 1 ), and MAPK . Thus elevation of the MAPK signaling pathway is required for arrest at metaphase II, and fertilization leads to the rapid degradation of MAPK. It should be emphasized that our understanding of normal oocyte maturation has had a major impact on the ability to treat infertile couples through the process of in vitro fertilization (IVF). Normal oocyte biology dictates the type of hormonal treatment, the timing of egg retrieval, and the meiotic stage of eggs used for fertilization.
Endocrine Function
Both theca and mural granulosa cells express LH receptors at the time of the LH surge. The LH surge induces terminal differentiation (called luteinization ) of the granulosa cells—a process that will continue for several days after ovulation. During the periovulatory period, the LH surge induces the following shifts in the steroidogenic activity of the mural granulosa cells ( Fig. 10.11 ):
- 1.
It transiently inhibits CYP19 expression and, consequently, estrogen production. The rapid decline in estrogen helps to turn off the positive feedback on LH secretion.
- 2.
It causes the direct vascularization of the granulosa cells by inducing the breakdown of the basal lamina. This makes LDL and HDL cholesterol accessible to these cells for steroidogenesis. The LH surge also increases the expression of the LDL receptor and HDL receptor (SR-BI) in granulosa cells.
- 3.
It increases the expression of StAR protein, CYP11A1 (side-chain cleavage enzyme), and 3β-HSD . Because CYP17 activity, especially the 17,20-lyase function, is largely absent in granulosa cells, these cells begin to secrete progesterone, and progesterone levels will gradually increase over the next week.