Objectives
- 1.
List the members of the three enteroendocrine hormone families: gastrin, secretin, and motilin.
- 2.
Describe how autonomic innervation and gastric and duodenal hormones regulate gastric acid secretion and gastric motility.
- 3.
Diagram the regulation of secretion from the exocrine pancreas and the gallbladder and their associated ducts from autonomic innervation and duodenal hormones.
- 4.
Explain how motilin regulates gastric and small intestinal contractions during the interdigestive period.
- 5.
Explain the role of enteroendocrine hormones in the regulation of gastrointestinal (GI) tract growth (an enterotropic action).
- 6.
Describe the role of glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) as incretin hormones.
Note: A fifth general function of GI hormones, the effect on appetite, is discussed in the context of energy homeostasis in Chapter 3 .
We begin our discussion of endocrine physiology with the hormonal function and regulation of the gastrointestinal (GI) tract. The discovery of secretin in 1902 by Bayliss and Starling represented the first characterization of a hormone as a blood-borne chemical messenger, released at one site and acting at multiple other sites. Indeed, the epithelial layer of the mucosa of the GI tract harbors numerous enteroendocrine cell types , which collectively represent the largest endocrine cell mass in the body.
The diffuse enteroendocrine system is perhaps the most basic example of endocrine tissue in that it is composed of unicellular glands situated within a simple epithelium. Most enteroendocrine cells, called open cells , extend from the basal lamina of this epithelium to the apical surface ( Fig. 2.1 ), although there are also closed enteroendocrine cells , which do not extend to the luminal surface. The apical membranes of open enteroendocrine cells express either receptors or transporters that allow the cell to sample the contents of the lumen. Luminal contents, called secretogogues , stimulate specific enteroendocrine cell types to secrete their hormones. This sampling or nutrient tasting is independent of osmotic and mechanical forces. The secretogogue mechanisms involved are poorly understood, but some appear to require the absorption of the nutrient. There is also evidence for the luminal secretion of paracrine peptide factors from the surrounding absorptive epithelial cells that stimulate hormonal release from enteroendocrine cells. As part of their response to luminal contents, specific enteroendocrine cell types display distinct localizations along the GI tract ( Table 2.1 ). We will see that these localizations are central to the regulation and function of each cell type.

| Stomach | Duodenum | Jejunum | Ileum | Colon | |
|---|---|---|---|---|---|
| G cell (gastrin) | x | (x) | |||
| S cell (secretin) | x | x | |||
| I cell (CCK) | x | x | (x) | ||
| K cell (GIP) | x | x | |||
| L cell (GLP-1) | x | x | |||
| L cell (GLP-2) | x | x | |||
| M cell (motilin) | x | x | |||
| Ghrelin-secreting cell | x | (x) | (x) | (x) | (x) |
In the simplest model of enteroendocrine cell function, a hormone is released from the basolateral membrane in response to the presence of a secretogogue at the luminal side of the cell. The secreted hormone diffuses into blood vessels in the underlying lamina propria, thereby gaining access to the general circulation. Circulating GI hormones regulate GI tract functions by binding to specific receptors at one or more sites within the GI tract and its extramural glands. In the classic model, the secretion of the hormone by an enteroendocrine cell is subsequently terminated when the luminal concentration of its secretogogue diminishes, thereby terminating the secretion of the hormone.
This simple model of the enteroendocrine system does not fully account for the integration with other systemic responses to a meal. Both open and closed enteroendocrine cells are regulated by the enteric nervous system (ENS) and paracrine factors secreted by neighboring epithelial cells (intrinsic regulators of enteroendocrine cell function) . Additionally, there are extrinsic regulators of enteroendocrine cells , most notably the autonomic nervous system and endocrine glands that reside outside of the GI tract. Conversely, GI hormones can have local (i.e., paracrine) actions on the afferent nerves of autonomic or enteric reflexes , so the response to a GI hormone can be mediated by a neurotransmitter. Thus GI tract function is orchestrated through a complex interplay of neural and endocrine responses and actions. It is not surprising, therefore, that GI function is often perturbed in patients with psychiatric disorders (e.g., depression) and endocrine disorders (e.g., hyperthyroidism).
The hormones secreted by the enteroendocrine system function to maintain the health of the GI tract and its extramural glands and provide an integrated response to the acquisition of nutrients. This integrated response to GI hormones is due, in part, to their ability to regulate multiple functions of the GI tract.
Enteroendocrine Hormone Families and their Receptors
All established GI hormones are peptides and bind to G-protein-coupled receptors ( GPCRs ; see Chapter 1 ) located on the plasma membrane of target cells. GI hormones, as well as their cognate GPCRs, can be organized into gene families based on structural homologies. In this chapter, we discuss members of three enteroendocrine hormone families: gastrin, secretin , and motilin ( Table 2.2 ).
| Hormone family | Members of family | Receptor and primary signaling pathway | Primary distribution of the receptor (related to GI function) |
|---|---|---|---|
| Gastrin | Gastrin (G cell) | CCK2 receptor Gq – ↑ in Ca 2+ and PKC | Gastric ECL cell and parietal cell |
| CCK (I cell) | CCK1 receptor Gq – ↑ in Ca 2+ and PKC | Gallbladder muscularis and sphincter of hepatopancreatic ampulla Pancreatic acinar cells Pancreatic ducts Vagal afferents and enteric neurons Stomach muscularis and pyloric sphincter Gastric D cells | |
| Secretin | Secretin (S cell) | Secretin receptor Gs – ↑ in cAMP | Pancreatic ducts and biliary ducts Pancreatic acinar cells G cells and pancreatic cells |
| GLP-1 (L cell) | GLP-1 receptor Gs – ↑ in cAMP | β Cells of pancreatic islets | |
| GLP-2 (L cell) | GLP-2 receptor Gs – ↑ in cAMP | GI tract, especially small intestine | |
| GIP (K cell) | GIP receptor Gs – ↑ in cAMP | β Cells of pancreatic islets Gastric mucosa and muscularis | |
| Motilin | Motilin (M cell) | Motilin receptor Gq – ↑ in Ca 2+ and PKC (also binds erythromycin) | Stomach and small intestines, especially in smooth muscle cells and enteric neurons |
| Ghrelin (P/D1 cell) | GHS receptor type 1a (GHS-RIa) Gq – ↑ in Ca 2+ and PKC | Pituitary and hypothalamus |
The gastrin family includes gastrin and cholecystokinin (CCK) , which share a common stretch of 5 amino acids at the C-terminus. Gastrin binds with high affinity to the CCK-2 receptor (previously called the CCK-B/gastrin receptor ). CCK binds with high affinity to the CCK-1 receptor . Receptors of this family are Gq-coupled receptors that are linked to Ca 2+ and diacylglycerol (DAG)/PKC-signaling pathways.
The secretin family includes the hormones secretin , glucagon , and glucagon-like peptides ( including GLP-1 and GLP-2 ) and gastric inhibitory polypeptide (GIP; more recently referred to as glucose-dependent insulinotropic peptide —see later). This family also includes the neurocrine factor, vasoactive intestinal peptide (VIP) . The corresponding GPCRs for each member of the secretin family of peptides are also structurally related. These receptors are all primarily coupled to Gs signaling pathways that increase intracellular cyclic adenosine monophosphate (cAMP) in target cells.
The motilin family includes the hormones motilin and ghrelin . Ghrelin was originally identified as a growth hormone secretogogue (GHS) but is most abundant in the fundus of the stomach. The receptors for motilin and ghrelin are GPCRs that are linked to Gα-q/phospholipase/IP 3 pathways , which, in turn, stimulate protein kinase C- and Ca 2+ -dependent signaling pathways (see Chapter 1 ).
Many GI peptides are also expressed by tissues outside of the GI tract. Pathophysiologically, GI peptides can be secreted in an uncontrolled manner from tumors. Other physiologic sites of production include other endocrine glands (e.g., the pituitary gland) and reproductive structures. Several peptides are produced by the central (CNS) and peripheral (PNS) nervous systems, where they are used as neurotransmitters or neuromodulatory factors. For example, CCK is expressed in the neocortical region of the CNS and the genitourinary-associated nerves of the PNS. As for its role in the CNS, CCK has been linked to anxiety and panic disorders. This also means that receptors for these peptides also reside within the CNS, the PNS, and probably other nonneural tissues. Thus a pharmacologic agent (agonist or antagonist) related to a specific GI peptide can potentially have a wide range of effects, depending on its stability and whether it can cross the blood-brain barrier. The possibility also exists that extra-GI sites of synthesis can “spill over” into the general circulation and affect GI function.
Gastrin and the Regulation of Gastric Function
The stomach acts as a food reservoir. People eat discontinuously and typically eat more at one sitting than their GI tract can process immediately. Thus the stomach holds the ingested food and gradually releases partially digested food (chyme) into the first part of the small intestine, the duodenum. The layers of the stomach wall carry out two basic functions: secretion and contraction/relaxation.
Overview of Regulation of Gastric Secretion and Motility
The innermost layer of the stomach wall, the gastric mucosa , contains glandular and surface mucus-producing epithelia and can be divided into proximal and distal segments. Two of the proximal portions of the stomach ( fundus and body ) contain the main gastric mucosal glands ( Fig. 2.2 ). Within these glands, the parietal cells secrete “hydrochloric acid” (HCl) , which is important for hydrolysis of macromolecules, activation of proenzymes, and the sterilization of ingested food. Parietal cells also secrete intrinsic factor , which is a glycoprotein required for the efficient absorption of vitamin B 12 .
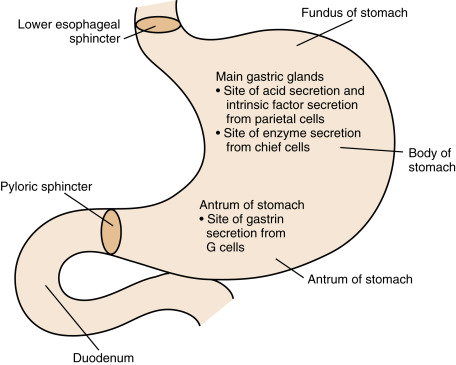
The glands of the fundus and body also contain the chief cells , which secrete digestive enzymes (e.g., pepsinogen, gastric lipase). A third cell type, the mucous cell , is found in the neck of the gastric glands and on the surface throughout the stomach. Mucous cells secrete mucigens, which buffer and protect the lining of the stomach, particularly in the vicinity of the main gastric glands. Because gastric enzyme and mucus production is primarily under nervous control, with little endocrine input, we focus here on gastric acid secretion and motility.
The distal part of the gastric mucosa, the pyloric antrum , has an important enteroendocrine function. This part of the stomach contains two types of “open” enteroendocrine cells. The G cells secrete gastrin , a hormone, and the D cells secrete somatostatin , a paracrine factor. These two peptides act antagonistically in a negative feedback loop to regulate gastric blood flow, cell growth, secretion, and motility (see later). D cells are also found within the fundus and body region, where they directly inhibit parietal cell secretion.
An outer layer of the stomach wall, the muscularis externa, is composed of smooth muscle. The relaxation of this muscle allows distention and storage, and its contractions ultimately move the partially digested food ( chyme ) into the duodenum. There are two gateways into and out of the stomach. These are the lower esophageal sphincter (LES) and the pyloric sphincter, respectively. The LES allows swallowed food particles to enter the stomach and protects the esophagus from the reflux of acidic chyme. The pyloric sphincter operates in conjunction with the muscularis externa to allow only small particles of digested chyme to escape the stomach and enter the duodenum. The pyloric sphincter also prevents backflow of chyme into the stomach.
In general, regulation of gastric function involves the stimulation of secretion and motility as needed (i.e., in the presence of food), and the inhibition of gastric secretion and motility as acidic chyme reduces the pH of the stomach, or as chyme moves into the small intestine and colon. In this way, the stomach avoids excessive acid secretion in the absence of buffering foodstuffs. Further, the portion of the GI tract below the stomach protects itself from exposure to excessive amounts of acid, which is both damaging to the intestinal lining and inhibitory to the activity of intestinal enzymes. Additionally, the small intestine, in which the majority of digestion and absorption occurs, controls the flow rate of food into and through the small intestine to optimize digestion and absorption of nutrients, salts, and water. The inability to properly regulate acid secretion and its flow into the intestine usually gives rise to duodenal ulcers, although patients with a gastrin-producing tumor (Zollinger-Ellison syndrome) can present with ulceration of the esophagus, stomach, and duodenum.
The general model of gastric control in response to a meal can be organized into three phases. The cephalic phase , which accounts for about 20% of the response to a meal during the digestive period, is activated by the actual or imagined smell and sight of food, or by the presence of food in the mouth. The cephalic phase is associated with increased gastric secretion but decreased motility, in anticipation of the need to store and start digesting food. The gastric phase , which accounts for about 10% of the postprandial response, is activated by the presence of food in, and mechanical distention of, the stomach. During the gastric phase, secretion is strongly stimulated, and this is accompanied by an increase in peristaltic contractions and gastric emptying. The third phase is the intestinal phase , during which an acidic mixture of partially digested food (chyme) moves in a regulated manner through the pyloric sphincter into the small intestine and ultimately into the colon. The processes of enzymatic digestion and absorption that occur during the digestive phase account for 70% of the digestive period. The movement of food into the lower GI tract generally moderates both gastric secretion and emptying.
Gastrin and the Stimulation of Gastric Function
Gastric HCl secretion from parietal cells is stimulated by three pathways:
- •
Paracrine stimulation by histamine, which is secreted by neighboring enterochromaffin-like (ECL) cells that reside within the lamina propria of the mucosa
- •
ENS and vagal parasympathetic nervous system stimulation via gastrin-releasing peptide (GRP) and acetylcholine
- •
Direct and indirect hormonal stimulation by the peptide hormone gastrin
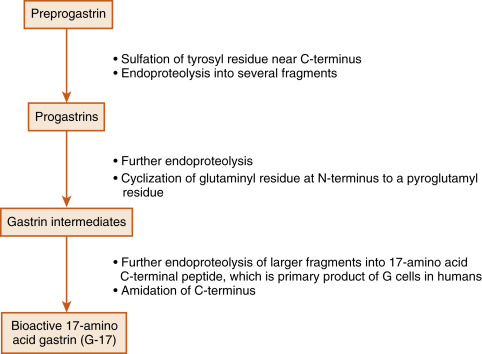
Gastrin is produced by the G cells of the stomach antrum and proximal duodenum. In humans, the term gastrin refers to a 17-amino acid peptide that has modifications at both termini ( G-17 ). In fact, the production of G-17 is an excellent example of how a peptide-encoding gene gives rise to multiple, larger precursors, which are also secreted into the blood. G-17 is the product of sequential posttranslational processing of preprogastrin , which can be generally characterized in three phases ( Fig. 2.3 ). In the first phase, sulfation and proteolysis generate a mixture of gastrin precursors, called progastrins. The second phase involves proteolysis within secretory granules that generates C-termini peptides. Processing of these intermediates also includes the cyclization of the glutaminyl to a pyroglutamyl residue. The third stage involves the amidation of the C-terminus to produce amidated gastrins. The primary secreted bioactive product of human G cells is G-17 (i.e., 17 amino acids). The pyroglutamyl residue at the amino terminus and the amidation of the C-terminus protect G-17 from digestion by circulating aminopeptidases and carboxypeptidases. G-17 binds with high affinity to the CCK2 receptor and is responsible for all of the gastrin effects on the stomach. The last four amino acids assign gastrin-like biologic activity to G-17. A synthetic, clinically used form of gastrin, pentagastrin , contains the last four amino acids, plus an alanine at the amino terminus that confers increased stability.