Objectives
- 1.
Discuss the anatomy of the adrenal gland, including the vascular supply and cortical zonation.
- 2.
Diagram the synthesis and regulated release of catecholamines in the chromaffin cell.
- 3.
Explain the action of catecholamines on different adrenergic receptors and the integrated effects of catecholamines during exercise.
- 4.
Outline the differences between the steroidogenic pathways in each zone of the adrenal cortex.
- 5.
Describe the physiologic actions of cortisol, aldosterone, dehydroepiandrosterone sulfate (DHEAS), and other adrenal androgens.
- 6.
Describe the regulation of the zona fasciculata and zona reticularis by the pituitary.
- 7.
Describe the regulation of the zona glomerulosa by the renin–angiotensin II system.
- 8.
Describe the pathophysiology of adrenal hormone excess and underproduction.
In the adult, the adrenal glands emerge as fairly complex endocrine structures ( Box 7.1 ) that produce two structurally distinct classes of hormones: steroids and catecholamines . The catecholamine hormone, epinephrine , acts as a rapid responder to stresses such as hypoglycemia and exercise to regulate multiple parameters of physiology, including energy metabolism and cardiac output. Stress is also a major secretogogue of the longer-acting steroid hormone, cortisol, which regulates glucose use, immune and inflammatory homeostasis, cardiovascular tone, and numerous other processes. The adrenal glands also regulate salt and volume homeostasis through the steroid hormone, aldosterone. The adrenal gland secretes a large amount of the androgen precursor, dehydroepiandrosterone sulfate (DHEAS), which plays a major role in fetoplacental estrogen synthesis and as a substrate for peripheral androgen synthesis in women.
The adrenal gland is a hybrid gland consisting of a cortex and a medulla. The hormones of the adrenal gland are important regulators of metabolism and serve an important role in adaptation to stress. The hormone aldosterone is critical to normal salt balance and, hence, water balance. Because of the antiinflammatory and immunosuppressive actions of adrenal corticosteroids, synthetic analogs are widely used in the treatment of disorders ranging from skin rashes to arthritis.
Anatomy
The adrenal glands are bilateral structures located immediately superior to the kidneys ( ad, toward; renal, kidney) ( Fig. 7.1A ). In humans, they are also referred to as the suprarenal glands because they sit on the superior pole of each kidney. The adrenal glands are similar to the pituitary, in that they are derived from both neuronal tissue and epithelial (or epithelial-like) tissue. The outer portion of the adrenal gland, called the adrenal cortex , develops from mesodermal cells in the vicinity of the superior pole of the developing kidney. These cells form cords of epithelioid endocrine cells. The cells of the cortex develop into steroidogenic cells and produce mineralocorticoids, glucocorticoids , and adrenal androgens ( Fig. 7.2 ; see Fig. 7.1C ).

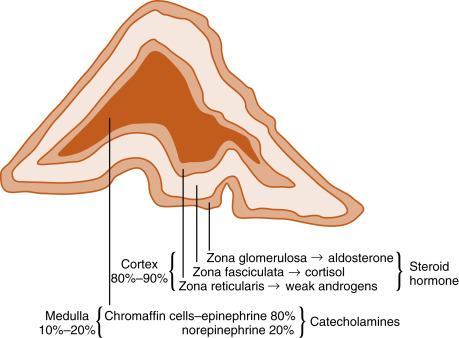
Soon after the cortex forms, neural crest–derived cells that are associated with the sympathetic ganglia—called chromaffin cells because they stain with chromium stains—migrate into the cortical cells and become encapsulated by them. Thus the chromaffin cells establish the inner portion of the adrenal gland, which is called the adrenal medulla (see Fig. 7.1C ). The chromaffin cells of the adrenal medulla have the potential of developing into postganglionic sympathetic neurons. They are innervated by cholinergic preganglionic sympathetic neurons and can synthesize the catecholamine neurotransmitter, norepinephrine , from tyrosine. However, the cells of the adrenal medulla are exposed to high local concentrations of cortisol from the cortex. Cortisol inhibits neuronal differentiation of the medullary cells so that they fail to form dendrites and axons. Additionally, cortisol induces the expression of an additional enzyme, phenylethanolamine-N-methyl transferase (PNMT) , in the catecholamine biosynthetic pathway. This enzyme adds a methyl group to norepinephrine, producing the catecholamine hormone, epinephrine , which is the primary hormonal product of the adrenal medulla (see Fig. 7.2 ).
The high local concentration of cortisol in the medulla is maintained by the vascular configuration within the adrenal gland. The outer connective tissue capsule of the adrenal gland is penetrated by a rich arterial supply coming from three main arterial branches (i.e., the inferior, middle, and superior suprarenal arteries; see Fig. 7.1A ). These give rise to the following two types of blood vessels that carry blood from the cortex to the medulla (see Fig. 7.1B ):
- 1.
Relatively few medullary arterioles that provide high oxygen and nutrient blood directly to the medullary chromaffin cells
- 2.
Relatively numerous cortical sinusoids, into which cortical cells secrete steroid hormones (including cortisol)
Both vessel types fuse to give rise to the medullary plexus of vessels that ultimately drain into a single suprarenal vein. Thus secretions of the adrenal cortex percolate through the chromaffin cells, bathing them in high concentrations of cortisol before leaving the gland.
Adrenal Medulla
Together, the two adrenal medullae weigh about 1 g. As described, the adrenal medulla is similar to a sympathetic ganglion without postganglionic processes. Instead of being secreted near a target organ and acting as neurotransmitters, adrenomedullary catecholamines are secreted into the blood and act as hormones. About 80% of the cells of the adrenal medulla secrete epinephrine , and the remaining 20% secrete norepinephrine . Although circulating epinephrine is derived entirely from the adrenal medulla, only about 30% of the circulating norepinephrine comes from the medulla. The remaining 70% is released from postganglionic sympathetic nerve terminals and diffuses into the vascular system. Because the adrenal medulla is not the sole source of catecholamine production, this tissue is not essential for life.
Synthesis of Epinephrine
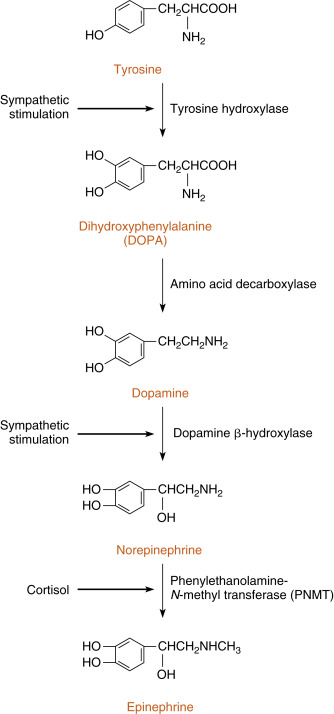
The enzymatic steps in the synthesis of epinephrine are shown in Fig. 7.3 . Synthesis begins with the sodium-linked transport of the amino acid, tyrosine , into the chromaffin cell cytoplasm and the subsequent hydroxylation of tyrosine by the rate-limiting enzyme, tyrosine hydroxylase , to produce dihydroxyphenylalanine (DOPA). DOPA is converted to dopamine by the cytoplasmic enzyme, aromatic amino acid decarboxylase, and is then transported into the secretory vesicle (also called the chromaffin granule ). Within the granule, dopamine is converted to norepinephrine by the enzyme, dopamine β-hydroxylase. This is an efficient reaction, so essentially all of chromaffin granule dopamine is converted to norepinephrine. In most adrenomedullary cells, essentially all of the norepinephrine diffuses out of the chromaffin granule by facilitated transport and is methylated by the cytoplasmic enzyme, PNMT , to form epinephrine. Epinephrine is then transported back into the granule by vesicular monoamine transporters (VMATs).
Multiple molecules of epinephrine, and to a lesser extent norepinephrine, are stored in the chromaffin granule complexed with adenosine triphosphate (ATP), Ca 2+ , and proteins called chromogranins . These multimolecular complexes are thought to decrease the osmotic burden of storing individual molecules of epinephrine within chromaffin granules. Chromogranins play a role in the biogenesis of secretory vesicles and the organization of components within the vesicles. Circulating chromogranins can be used as a marker of sympathetic paraganglion-derived tumors (paragangliomas). Chromaffin cells also synthesize several secretory peptides, including adrenomedullin and enkephalins, which can have local, subtle effects on sympathetic input and adrenomedullary response.
Secretion of epinephrine and norepinephrine from the adrenal medulla is regulated primarily by descending sympathetic signals in response to various forms of stress, including emotional stress (fear, anger), exercise, hypoglycemia, and surgery. The primary autonomic centers that initiate sympathetic responses reside in the hypothalamus and brainstem, and they receive inputs from the cerebral cortex, the limbic system, and other regions of the hypothalamus and brainstem.
The chemical signal for catecholamine secretion from the adrenal medulla is acetylcholine (ACh) , which is secreted from preganglionic sympathetic neurons and binds to nicotinic receptors on chromaffin cells. Nicotinic receptors lead to depolarization of the chromaffin cell membrane followed by opening of voltage sensitive Ca 2+ channels. ACh signaling increases the activity of the rate-limiting enzyme, tyrosine hydroxylase, in chromaffin cells (see Fig. 7.3 ). ACh also increases the activity of dopamine β-hydroxylase and stimulates exocytosis of the chromaffin granules. Synthesis of epinephrine and norepinephrine is closely coupled to secretion so that the levels of intracellular catecholamines do not change significantly, even in the face of changing sympathetic activity. As discussed earlier, cortisol regulates epinephrine production by maintaining adequate expression of the PNMT gene in chromaffin cells (see Fig. 7.3 ).
Mechanism of Action of Catecholamines
Catecholamines act through membrane GPCRs (see Chapter 1 ). The individual types of adrenergic receptors were first classified based on their pharmacology, and this classification scheme has been supported by genetics and molecular cloning. Adrenergic receptors are generally classified as α- and β-adrenergic receptors, and these are further divided into α 1 and α 2 receptors and β 1 , β 2 , and β 3 receptors ( Table 7.1 ). These receptors can be characterized according to the following elements:
- 1.
The relative potency of endogenous and pharmacologic agonists and antagonists. For example, the synthetic isoproterenol is more potent than either epinephrine or norepinephrine on cardiac β 1 adrenergic receptors. A large number of synthetic selective and nonselective adrenergic agonists and antagonists now exist.
- 2.
Downstream signaling pathways. Table 7.1 shows the primary pathways that are coupled to the different adrenergic receptors. This is an oversimplification because differences in signaling pathways for a given receptor have been linked to duration of agonist exposure and cell type.
- 3.
Location and relative density of receptors. Importantly, different receptor types predominate in different tissues. For example, although both α 2 and β 2 receptors are expressed by islet β cells, the predominant response to a sympathetic discharge is mediated by α 2 receptors.
| Receptor Type | Primary Mechanism of Action | Examples of Tissue Distribution |
|---|---|---|
| α 1 | ↑IP 3 , DAG | Vascular smooth muscle |
| α 2 | ↓cAMP | Pancreatic β cells |
| β 1 | ↑cAMP | Heart |
| β 2 | ↑cAMP | Liver |
| β 3 | ↑cAMP | Adipose |
Physiologic Actions of Adrenomedullary Catecholamines
Because the adrenal medulla is directly innervated by the autonomic nervous system, adrenomedullary responses are very rapid. Because of the involvement of several centers in the central nervous system (CNS), most notably the cerebral cortex, adrenomedullary responses can precede onset of the actual stress (i.e., they can be anticipated). In many cases, the adrenomedullary output, which is primarily epinephrine, is coordinated with sympathetic nervous activity as determined by the release of norepinephrine from postganglionic sympathetic neurons. However, some stimuli (e.g., hypoglycemia) evoke a stronger adrenomedullary response than a sympathetic nervous response, and vice versa.
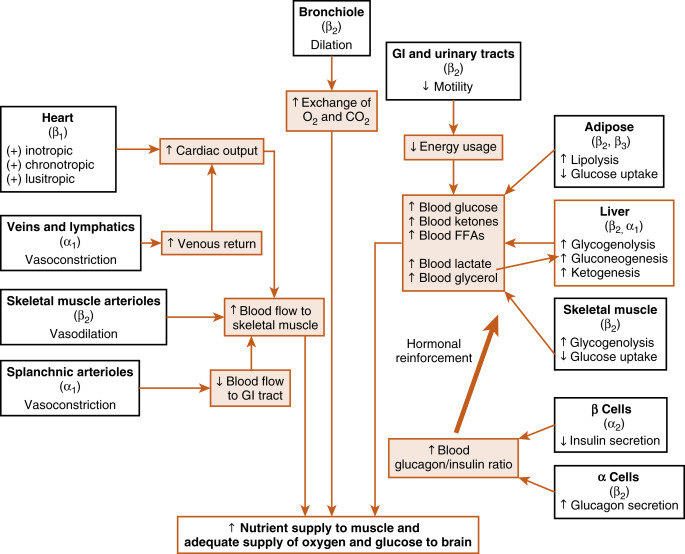
Many organs and tissues are affected by a sympathoadrenal response. An informative example of the major physiologic roles of catecholamines is the sympathoadrenal response to exercise. Exercise is similar to the fight-or-flight response, but without the subjective element of fear. Exercise increases circulating levels of both norepinephrine and epinephrine. The overall goal of the sympathoadrenal system during exercise is to meet the increased energy demands of skeletal and cardiac muscle while maintaining sufficient oxygen and glucose supply to the brain. The response to exercise includes three of the following major physiologic actions of norepinephrine and epinephrine ( Fig. 7.4 ):
- 1.
Increased blood flow to the muscles is achieved by the integrated actions of norepinephrine and epinephrine on the heart, veins, and lymphatics, and the nonmuscular (e.g., splanchnic) and muscular arteriolar beds. Norepinephrine and epinephrine act on β 1 receptors at the heart to increase the rate (chronotropy) and strength (inotropy) of contractions and facilitate ventricular relaxation during diastole (lusitropy). Catecholamines also induce vasoconstriction through α-adrenergic receptors of high-capacity vessels (veins and lymphatics), thereby increasing venous return to the heart. All these effects increase cardiac output . Catecholamines shunt blood away from the gastrointestinal (GI) tract through vasoconstriction of splanchnic arterioles (α receptors) and increase blood flow to skeletal muscle by inducing vasodilation of muscle arteriolar beds through β 2 receptors.
- 2.
Epinephrine promotes glycogenolysis in muscle through β 2 receptors. Exercising muscle can also use free fatty acids (FFAs), and epinephrine and norepinephrine act through β 2 and β 3 receptors, respectively, to promote lipolysis in adipose tissue. The actions just described increase circulating levels of lactate and glycerol, which can be used by the liver as gluconeogenic substrates to increase glucose. Epinephrine does, in fact, increase blood glucose by increasing hepatic glycogenolysis and gluconeogenesis through β 2 receptors. The promotion of lipolysis in adipose tissue is also coordinated with an epinephrine-induced increase in hepatic ketogenesis . Finally, the effects of catecholamines on metabolism are reinforced by the fact that they stimulate glucagon secretion (β 2 receptors) and inhibit insulin secretion (α 2 receptors). Efficient production of ATP during normal exercise (i.e., a 1-hr workout) also requires efficient exchange of gases with an adequate supply of oxygen to exercising muscle. Epinephrine promotes this by relaxation of bronchiolar smooth muscle through β 2 receptors.
- 3.
Catecholamines decrease energy demand by visceral smooth muscle. In general, a sympathoadrenal response decreases overall motility of the smooth muscle in the GI tract and urinary tract, thereby conserving energy where it is not needed.
Metabolism of Catecholamines
In general, the duration of circulating epinephrine actions is longer than those of norepinephrine released from an autonomic neuron. There are two primary enzymes involved in the degradation of catecholamines: monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT). Although MAO is the predominant enzyme in neuronal mitochondria, both enzymes are found in many nonneuronal tissues, including liver and kidney. The neurotransmitter norepinephrine is degraded by MAO and COMT after uptake of the compound into the presynaptic terminal. This mechanism is also involved in the catabolism of circulating adrenal catecholamines. However, the predominant fate of adrenal catecholamines is methylation by COMT in nonneuronal tissues such as the liver and kidney. The metabolism of catecholamines is shown in Fig. 7.5 . Urinary vanillylmandelic acid (VMA) and metanephrine are sometimes used clinically to assess the level of catecholamine production in a patient. Much of the urinary VMA and metanephrine is derived from neuronal, rather than adrenal, catecholamines.
A pheochromocytoma is a neuroendocrine tumor that produces excessive quantities of epinephrine and norepinephrine. Paragangliomas are derived from nonadrenal sympathetic ganglia and secrete only norepinephrine. Although pheochromocytomas are not common tumors, they are the most common source of hyperadrenal medullary function and are often used as an example to demonstrate the functions of the adrenal medulla ( Box 7.2 ). For unknown reasons, the symptoms of excessive catecholamine secretion are often sporadic rather than continuous. The symptoms include hypertension, headaches (from hypertension), sweating, anxiety, palpitations, and chest pain. In addition, patients with this disorder may experience orthostatic hypotension (despite the tendency for hypertension). This occurs because hypersecretion of catecholamines can decrease the postsynaptic response to norepinephrine as a result of downregulation of the receptors. Consequently, the baroreceptor response to the blood shifts that occur on standing is blunted.
- •
Metabolic
- •
Hyperglycemic
- •
Glycogenic
- •
Gluconeogenic
- •
Lipolytic
- •
Protein catabolic
- •
Insulin antagonist in muscle and adipose tissue
- •
Inhibits bone formation, stimulates bone resorption
- •
Necessary for vascular response to catecholamines
- •
Antiinflammatory
- •
Suppresses immune system
- •
Inhibits antidiuretic hormone secretion and action
- •
Stimulates gastric acid secretion
- •
Necessary for integrity and function of gastrointestinal tract
- •
Stimulates red blood cell production
- •
Alters mood and behavior
- •
Permissive for calorigenic, lipolytic effects of catecholamine
Adrenal Cortex
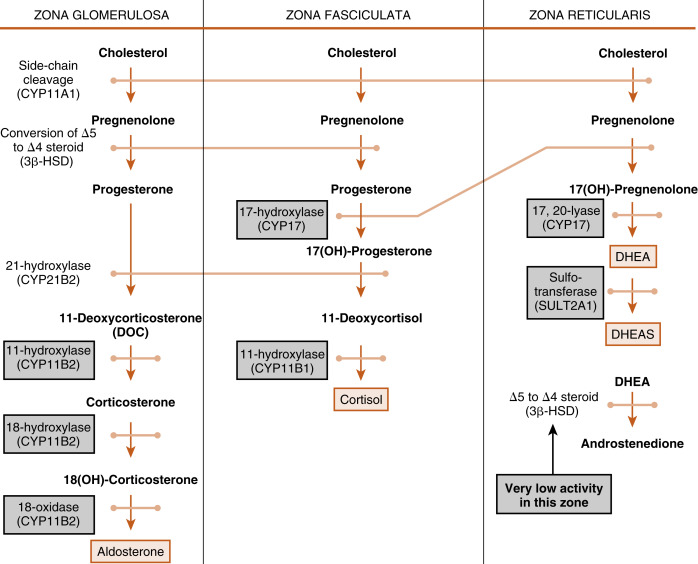
The cortex of the adult human adrenal shows distinct zonation with respect to histologic appearance, steroidogenesis, and regulation. The adrenal cortex is made up of three zones: the outer zona glomerulosa, the middle zona fasciculata, and the inner zona reticularis (see Fig. 7.2 ). Each zone expresses a distinct complement of steroidogenic enzymes, resulting in the production of a different steroid hormone as the major endocrine product for each zone as summarized in Fig. 7.5 . Recall from Chapter 1 that steroid hormones are derived from cholesterol , which is enzymatically modified in a cell type–specific manner. This means that the steroidogenic endocrine cells are characterized by the steroidogenic enzymes they express, as well as their final hormonal product. Associated with the production of a different steroid hormone, each zone has unique aspects concerning its regulation and the configuration of feedback loops. Recall also from Fig. 1.5 ( Chapter 1 ) that the last steroid molecule within a steroidogenic pathway is what leaves the cell and enters the blood. This means that in the face of a loss of a steroidogenic enzyme within a pathway, the primary steroidlike product released from the gland is the substrate for that missing enzyme, because all subsequent reactions of the pathway cease to occur. An understanding of the steroidogenic pathways for each steroid hormone and steroidogenic cell type is required to understand the consequences of specific mutations in genes encoding steroidogenic enzymes and in states of dysregulation of specific steroidogenic pathways.
Zona Fasciculata
The Zona Fasciculata Makes Cortisol
The largest and most actively steroidogenic zone is the middle zona fasciculata (see Figs. 7.1B and 7.2 ). The zona fasciculata produces the glucocorticoid hormone, cortisol . This zone is composed of straight cords of large cells. These cells have a foamy cytoplasm because they are filled with lipid droplets that represent stored cholesterol esters . Although the cells make some cholesterol de novo from acetate, they are very efficient at capturing cholesterol from the blood circulating in the form of low-density lipoprotein (LDL) particles (delivery by high-density lipoprotein [HDL] is minimal in humans). Free cholesterol is then esterified by the enzyme acyl CoA cholesterol transferase (ACAT) and stored in lipid droplets ( Fig. 7.6 ). The stored cholesterol is continually turned back into free cholesterol by hormone-sensitive lipase (HSL), a process that is increased by adrenocorticotropic hormone (ACTH; see later in the text).
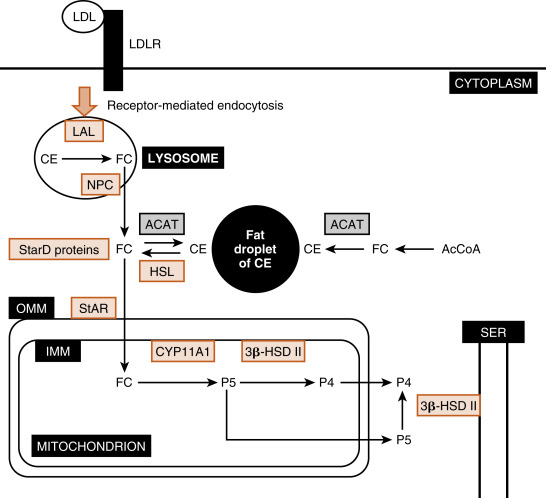
Free cholesterol is modified by five reactions within a steroidogenic pathway to form cortisol. However, cholesterol is stored in the cytoplasm, and the first enzyme of the pathway, CYP11A1 , is located on the inner mitochondrial membrane (see Fig. 7.6 ). Thus the rate-limiting reaction in steroidogenesis is the transfer of cholesterol from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane and its conversion to pregnenolone (P5) . Although several proteins appear to be involved, one protein, called steroidogenic acute regulatory protein (StAR protein) , is indispensable in the process of transporting cholesterol to the inner mitochondrial membrane (see Fig. 7.6 ). StAR is associated with the OMM and phosphorylation by ACTH-Gs-cAMP-PKA signaling increasing StAR activity.
Endocytosed LDL particles are enzymatically digested by lysosomal enzymes. Free cholesterol, but not cholesterol esters, is transported out of the lysosome and enters the cellular cholesterol pool. Cholesterol esters are cleaved by lysosomal acid lipase (LAL) encoded by the LIPA gene. Mutations in the LIPA gene cause cholesterol ester storage disease and the more severe variant, Wolman disease . Wolman disease affects numerous organs and is ultimately fatal. With respect to the adrenal cortex, Wolman disease causes adrenal insufficiency due to the inability of cells to use LDL cholesterol for steroidogenesis. This underscores the importance of LDL cholesterol for steroidogenesis.
The Niemann-Pick disease C transporters (NPC1 and NCP2) are required for transport of free cholesterol out of the lysosome after receptor-mediated endocytosis of the LDL receptor. NPC disease is caused by a mutation in either the NPC1 gene or, at a much lower frequency, the NPC2 gene. NPC disease leads to progressive neurodegeneration and death within the first decade of life.
StAR protein is encoded by the StarD1 gene. Inactivating mutations in StarD1 cause cells of the adrenal cortex and gonads to become excessively laden with lipid (“lipoid”) because cholesterol cannot be accessed by CYP11A1 within the mitochondria and used for hormone synthesis. Loss of cortisol increases ACTH, causing adrenal hypertrophy. Thus mutations in StarD1 lead to lipoid congenital adrenal hyperplasia . Elevated ACTH also increases cholesterol synthesis and transport of cholesterol into the cell cytoplasm through LDL receptor–mediated endocytosis, worsening the engorgement of the cell with lipid. Affected individuals make a small amount of cortisol, aldosterone , or gonadal steroid hormones as a result of StAR-independent transport. Aldosterone insufficiency represents the most serious deficit because it leads to salt wasting, reduced blood volume, and hypotension (especially orthostatic). Hypoaldosteronism also causes hyperkalemia and metabolic acidosis (see later in the text). Hypocortisolism is especially a serious threat in the face of infection, trauma, surgery, or extended fasting (see later in the text).
The pathway by which cortisol is synthesized involves three enzymes that are not specific to the adrenal and two enzymes that are specifically adrenocortical in their expression. Four of these enzymes belong to the cytochrome P-450 monooxidase gene family and thus are referred to as CYP s. The fifth enzyme is 3β-hydroxysteroid dehydrogenase type 2 (3β-HSD2).
The steroidogenic pathway from cholesterol to cortisol is as follows (refer to Fig. 1.4 in Chapter 1 for structure of cholesterol and numbering of cholesterol carbons):
- •
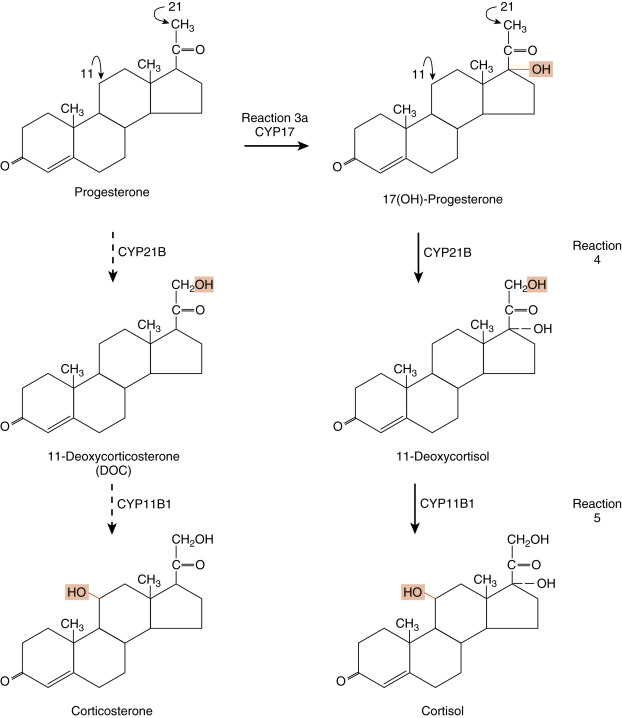
Reaction 1 . The side chain of cholesterol (carbons 22 to 27) is removed by CYP11A1 (also called P-450 side-chain cleavage ) to generate a C21 steroid intermediate, pregnenolone ( Fig. 7.7 ). Generating a C21 intermediate is a key step because cortisol (as well as aldosterone and progesterone) is a 21-carbon steroid.
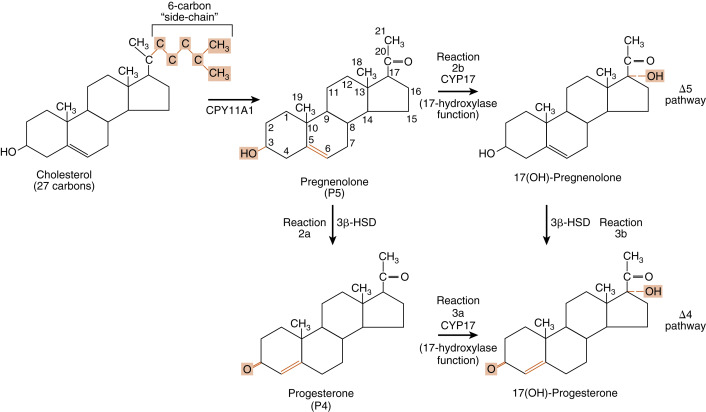
Fig. 7.7
Reaction 1, catalyzed by CYP11A1, in making cortisol. Reactions 2a/b and reactions 3a/b, involving CYP17 (17-hydroxylase function) and 3β-hydroxysteroid dehydrogenase (3β-HSD), in making cortisol.
- •
Reactions 2a/b and 3a/b . The next two enzymes compete with each other for pregnenolone, so they will be presented as reactions 2a and 2b. The products of reactions 2a and 2b are then modified by the reciprocal enzymes in reactions 3a and 3b, to generate the final product, 17-hydroxyprogesterone (see Fig. 7.7 ).
- •
Reaction 2a . Pregnenolone is a substrate for the enzyme, 3β-HSD2 , which converts the hydroxyl group on the 3 carbon to a ketone and moves the double bond from the 5 to 6 (Δ5) position to the 4 to 5 (Δ4) position. All active steroid hormones must be converted to Δ4 structures. This reaction converts pregnenolone (also called P5 , because it is a Δ5 steroid) to progesterone (also called P4 , because it is a Δ4 steroid).
- •
Reaction 3a. Progesterone is then hydroxylated to 17-hydroxyprogesterone by CYP17 . 17-Hydroxylation is an indispensable step for the formation of cortisol (and sex steroids). We will see that the presence or absence of CYP17 plays an important role in defining the nature of steroidogenic tissue.
- •
Reaction 2b . Pregnenolone can also be hydroxylated by CYP17 to 17-hydroypregnenolone (this is called the Δ5 pathway ). Because of the high level of 3β-HSD2 activity, reaction 2b is less important than reaction 2a (previous text.)
- •
Reaction 3b . 17-Hydroxypregnenolone can then be converted to 17-hydroxyprogesterone by 3β-HSD. Note that CYP17 has two separate activities: a 17-hydroxylase function , and a 17,20-lyase function . This latter function removes the 20 and 21 carbons, reducing the steroid to a 19-carbon precursor of active androgens. The zona fasciculata does not express cofactors that promote the 17,20-lyase activity of CYP17 and therefore does not produce significant amounts of androgen precursors. Instead, 17-hydroxyprogesterone is efficiently funneled into the cortisol-specific pathway, which involves two subsequent hydroxylations by adrenocortical-specific enzymes.
- •
Reactions 4 and 5 . 17-Hydroxyprogesterone is hydroxylated on the 21 carbon by CYP21 , producing 11-deoxycortisol . 11-Deoxycortisol is then efficiently hydroxylated on the 11 carbon by CYP11B1 , producing cortisol ( Fig. 7.8 ). Note that progesterone (the product of reaction 2a) can also enter this pathway of 21- and 11-hydroxylations, producing deoxycorticosterone (DOC) and corticosterone , respectively (see Fig. 7.5 ). However, CYP17 activity is robust in the human zona fasciculata, so DOC and corticosterone are normally minor products.