The dynamics of ICU-acquired HAIs are complex and depend on the contribution of the host’s underlying conditions, the infectious agents, and the unique environment of the ICU. The following discussion will consider the role of each component in the development of HAI.
Host Defenses
The ability of patients in ICUs to ward off infections is seriously compromised. Natural host defense mechanisms might be impaired by underlying diseases or as a result of medical and surgical interventions. All patients admitted to an ICU will have at least one, and often several, indwelling devices that break the normal skin barriers and establish direct access between the external environment and normally sterile body sites. Natural chemical barriers in the stomach are neutralized by administering H2-blockers or antacids that reduce acidity and allow growth of enteric flora. Physiologic mechanisms for evacuating and cleansing hollow organs are disrupted and circumvented by insertion of endotracheal tubes, nasogastric tubes, and urinary catheters.
Specific host defense mechanisms also might be impaired by the underlying diseases. Patients with malignant disorders might have abnormal immune responses as a result of their disease or stemming from therapies that diminish the number of effective phagocyte cells and blunt the normal immune response. Patients admitted to ICUs who are at the extremes of age exhibit selected impairments in natural and specific defense mechanisms that increase the HAI risk (
6,
7). A historical cohort study from Belgium revealed, however, that the incidence of healthcare-associated bloodstream infection (BSI) was lower among very old ICU patients when compared to middle-aged and old patients. Yet, the adverse impact of this HAI was higher in very old patients (
8).
Because of the precarious condition of patients in the ICU, normal food intake often is suspended, leading to under- or malnutrition (
9). Injured tissue, perfusion deficits, and infection cause fever and tachycardia through mechanisms mediated by hormones and cytokines, such as endotoxin. The physiologic response to these mediators is an increase in oxygen consumption stemming from an increase in metabolic demand. This response results in breakdown of muscle to meet the body’s demand for energy. The lean body mass declines, resulting in deficits in substrates necessary for recovery (
10).
Under-nutrition has been associated with increased complication rates and delayed wound healing (
11,
12). Several studies suggest that poor nutritional status is a predisposing factor for HAIs (
13,
14,
15). Recent studies have confirmed that the use of enteral nutrition vs. total parenteral nutrition (TPN), early initiation of enteral nutrition, and use of enteral and parenteral glutamine are all associated with reduced infectious morbidity in critically ill patients (
16,
17,
18). For instance, early or glutamineenriched enteral nutrition in critically ill patients has been reported to decrease HAIs and other complications (
19,
20). Conversely, a meta-analysis including 26 studies which examined the relationship between TPN and mortality rates in critically ill
patients showed that TPN had no effect on mortality and only lowered complication rates in malnourished patients (
21). In a meta-analysis of trials comparing enteral nutrition to TPN in ICU patients, Simpson et al. reported that TPN was even associated with an increase in infectious complications (odds ratio [OR] = 1.47; 95% confidence interval [CI] = 0.90 to 2.38) (
22). Two other recent meta-analyses reported negative findings. In their systematic review, Peter et al. reported no mortality effect with the type of nutritional supplementation, although early enteral nutrition significantly reduced complication rates (
23). Ho et al. performed a meta-analysis, comparing early gastric and postpyloric feeding in critically ill patients and concluded that early use of postpyloric feeding instead of gastric feeding in ICU patients with no evidence of impaired gastric emptying was not associated with significant clinical benefits (
24).
Important alterations in T- and B-cell function affecting host defense and resistance to infection are found in critically ill and traumatized patients (
25). Alterations in T-cell activation and cytokine production are frequently associated with trauma and hemorrhage. Injury and blood loss result in activation of CD8 T-cell populations capable of altering bacterial antigen-specific B-cell repertoires and suppressing the function of other T cells.
Systemic hypoxia and hypovolemia also are significant contributors to the development of infection. However, significant changes in perioperative care have been introduced in recent years (
26,
27). The maintenance or restoration of normal physiologic characteristics after surgery becomes the key to preventing complications (
28).
Medical Devices
The results of the first European Prevalence of Infection in Intensive Care (EPIC) study (
29) highlighted the relative importance of medical devices as risk factors for infections compared with other factors. Factors were collected from >10,000 ICU patients, of whom 2,064 had ICU-acquired infections. Among the seven independent risk factors identified, four were associated with medical devices commonly used in intensive care: central venous catheter (CVC) (OR = 1.35, 95% CI = 1.60 to 1.57), pulmonary artery catheter (OR = 1.20, 95% CI = 1.01 to 1.43), urinary catheter (OR = 1.41, 95% CI = 1.19 to 1.69), and mechanical ventilation (OR = 1.75, 95% CI = 1.51 to 2.03). Other independent risk factors for ICU-acquired infections were stress ulcer prophylaxis (OR = 1.38, 95% CI = 1.20 to 1.60), the presence of trauma on admission (OR = 2.07, 95% CI = 1.75 to 2.44), and the length of ICU stay. The latter constituted the strongest predictor of infection and showed a linear increase in the odds for infection with time spent in the ICU (
29). This finding also was confirmed by the second international study of the prevalence and outcomes of infection in ICUs, published in 2009 (
2).
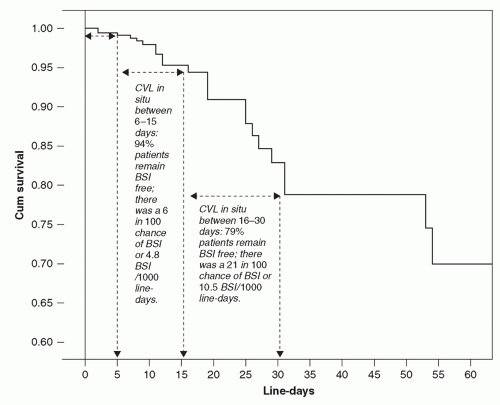
In another interesting cohort study (
30), McLaws and Berry analyzed the rate for CVC-associated BSI in 1,375 patients who were monitored for 7,467 days of CVC use. They found significant differences in the BSI rate depending on the length of catheterization (
Figure 24.1). The probability of BSI with a CVC in place was 6% by day 15, 14% by day 25, 21% by day 30, and 53% by day 320. Thus, the risk of infection is not homogenous, but increases substantially after prolonged CVC-insertion (>2 weeks).
Underlying Diseases
ICUs, by design, serve patients with severe illnesses that compromise host defense. Each patient must be assessed individually to determine how the underlying illness might interfere with host defense mechanisms. A simple assessment of the severity of underlying illness was developed five decades ago by McCabe and Jackson (
31), who stratified patients according
to whether the underlying disease was fatal, ultimately fatal, or nonfatal. Subsequent studies by Britt and colleagues (
32) have demonstrated the utility of this simple assessment for estimating the risk of nosocomial BSI. Numerous other studies have found increasing rates of infections among patients with more severe illness (
33,
34).
Although McCabe’s classification has been useful, it was not designed to assess patients admitted to ICUs. Therefore, several severity-of-illness scoring systems have been proposed to estimate a patient’s risk of death in ICUs objectively. Great progress has been observed in the last 10 years in the accuracy of statistical models to assess critically ill patients and predict survival (
35,
36). Customized or modified versions of the most frequently used scoring systems (e.g., simplified acute physiology score [SAPS] III; Acute Physiology, Age, and Chronic Health Evaluation [APACHE] III) have been proposed to obtain satisfactory estimates of the probability of death in ICU patients, which depends on the severity of illness, the number of acute organ failures, and the characteristics of underlying disease (
37,
38,
39,
40). Nevertheless, limitations persist about the capacity of these scoring systems to integrate differences in overall quality of care (
41). Moreover, older versions of these scores, which were developed in the early 1990s, have shown a decline in predictive accuracy as the models age. Therefore, mortality tends to get overpredicted when older models are applied to more contemporary data, which in turn leads to biased benchmarking data of different ICUs (
42). Thus, care should be applied when using outdated severity scoring models to contemporary populations.
A group of critical care physicians developed, by consensus, the so-called “Sepsis-Related Organ Failure Assessment” (SOFA) score in 1994, a severity scoring system that targets septic patients (
43). Since the score is not specific for sepsis, it was later called “Sequential Organ Failure Assessment.” The SOFA score is composed of scores from six organ systems, graded from 0 to 4 according to the degree of dysfunction. While primarily designed to describe morbidity, several analyses showed a relationship between the SOFA score and mortality and indicated also a good distribution of patients among the different score values (
44).
Antimicrobial Usage and Selection Pressure
Different types of epidemiological studies have been used to quantify the association between antibiotic exposure and resistance in critically ill patients (
45,
46,
47,
48,
49). These studies included outbreak reports, laboratory-based surveys, randomized trials, and prospective or retrospective cohort studies based on analyses of individual-patient-level data or aggregated data. The different methodological approaches are not mutually transposable, and the lack of uniformity makes the comparison of different studies difficult. For instance, the analysis of aggregated data may be limited by “ecologic bias,” which is the failure of group-level-effect estimates to reflect the biological effect of antibiotic use at the individual-patient level (
45). This bias is a result of the fact that, unlike individual-level studies, ecologic studies do not link individual outcome events to individual antibiotic exposure histories. Notwithstanding these difficulties, the majority of studies confirm that large differences exist in the pattern of antimicrobial usage and antimicrobial resistance, between different hospitals and ICUs. Usage of antimicrobials may show important variations between institutions facing similar prevalences of highly resistant organisms, confirming that efforts to control resistance should focus on both antimicrobial use and infection control practices (
50,
51,
52).
In ICUs, where antibiotics are used more frequently and in larger amounts than in almost any other unit in the hospital, antimicrobial resistance ensures the survival of some nosocomial pathogens (
53). The close proximity of patients facilitates transfer of resistant organisms from patient to patient (
54). However, the high prevalence of carriage of multidrug-resistant microorganisms does not automatically translate into higher overall HAI rates in the concerned services (
55,
56).
It is noteworthy that trends in the pathogens responsible for HAIs in the ICU have shown an increase in infections due to multiply-resistant gram-negative bacteria (e.g.,
Enterobacter spp.,
Acinetobacter baumannii) and fungi such as
Candida spp. (
2). The emergence of these pathogens is due, at least in part, to patterns of antibiotic use and selection pressure and to the development of antibiotic resistance among these isolates (
57). In a multicenter study by Meyer et al.(
48) performed between 2001 and 2008 in 53 German ICUs, the carbapenem use almost doubled despite no significant change in the total antibiotic use expressed as defined daily doses (DDD)/1,000 patient-days. The exponential increase of third-generation cephalosporin resistance in
Escherichia coli and other
Enterobacteriaceae reported in this study led to switching empirical therapy to carbapenems to treat infections, with subsequent emergence of carbapenem-resistant
Klebsiella pneumoniae, carbapenemase-producing gram-negative pathogens, and imipenem-resistant
A. baumannii as a direct consequence. This scenario will affect many ICUs around the world in the near future, although resistance trends and antibiotic consumption rates will still depend on different determinants, that is, ICU characteristics (medical, surgical, general), local antibiotic policies, and physicians’ level of education among others. The heterogeneity of antibiotic prescriptions within ICUs recorded by Meyer and colleagues seems to indicate that antimicrobial use can be improved also in ICU settings by shortening the duration of treatment or antibiotic prophylaxis without affecting patient outcome (
58).
In contrast to multidrug-resistant gram-negative bacteria, infection rates seem to have stabilized or declined for multidrug-resistant gram-positive bacteria (e.g., methicillinresistant
Staphylococcus aureus [MRSA], vancomycin-resistant enterococcus [VRE]) in ICUs in many high-income countries (
59,
60,
61,
62). For instance, Jain et al. (
60) evaluated the effectiveness of a quality improvement initiative in preventing the acquisition and spread of MRSA among nearly 2 million patient admissions, including data from 196 ICUs in the United States. During the intervention period—with increased attention to MRSA admission screening, contact precautions, hand hygiene, and emphasis on responsibility of all healthcare workers in prevention procedures—an important decrease in infections not only caused by MRSA, but also by other pathogens was observed (
60). However, others have questioned the effectiveness of the intervention and suggest that other non-documented factors may have contributed to the observed reduction in MRSA infection rates in the participating ICUs (
63). Despite this ongoing controversy, the positive development of reduced MRSA rates could potentially reduce the necessity of empiric gram-positive coverage in many ICUs, but clinicians seem to be reluctant to adapt their treatment patterns to lower MRSA rates (
64).