Infections of Burn Wounds
David W. Mozingo
Basil A. Pruitt Jr.
Infection, the risk of which is proportional to the extent of injury, continues to be the predominant determinant of outcome in thermally injured patients despite improvements in overall care in general and wound care in particular. The control of invasive burn wound infection through the use of effective topical chemotherapy, prompt surgical excision, and timely closure of the burn wound has resulted in unsurpassed survival rates. Even so, infection remains the most common cause of death in these severely injured patients.
ETIOLOGY OF BURN INJURY
Burns are estimated to affect 450,000 people in the United States annually (1). Of this number, ~45,000 patients require hospital admission, 20,000 to 25,000 of whom have injuries of such significance that care is best undertaken in a burn center. House and structure fires are responsible for >70% of the yearly 3,500 burn-related deaths, 75% of which result from smoke inhalation or asphyxiation and 25% of which are due to burns. However, these fires are responsible for only 4% to 5% of burn admissions. Injuries due to contact with flame or ignition of clothing are the most common cause of burns in adults, whereas scald burns are most common in children. The majority of patients sustain burns of such limited severity and extent (>80% of burns involve <20% of the body surface) that they can be treated on an outpatient basis. Approximately 170 to 230 patients per million population per year require hospital admission owing to the extent of their burns or to other complicating factors. Approximately 33% of patients who require in-hospital care have a major burn injury—as defined by the American Burn Association on the basis of burn size, causative agent, preexisting disease, and associated injuries—and should be treated in a tertiary care burn center (2).
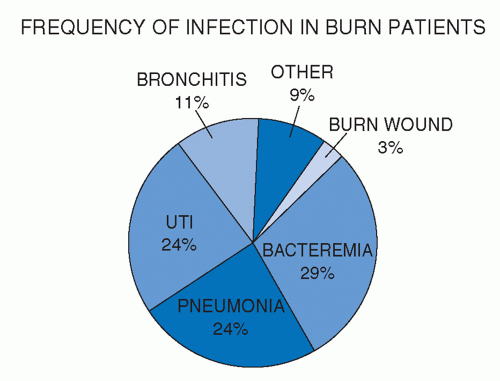
Changes in wound care over the past 40 years, including the use of effective topical antimicrobial chemotherapy and excision of the burned tissue to achieve timely closure of the burn wound, have significantly reduced the occurrence of invasive burn wound infection and its related morbidity and mortality. Regular collection of cultures from patients permits early identification of the causative pathogens of those infections that do arise. Moreover, infection control procedures, including strict enforcement of patient and staff hygiene and use of patient isolation precautions, have been effective in controlling the spread of multidrug-resistant organisms and eliminating them from the burn center. These advances and the improvements in the general care of critically ill burn patients have resulted in markedly improved survival rates. However, as a manifestation of the systemic immunosuppressive effects of burn injury, infection at other sites, predominantly the lungs, remains the most typical cause of morbidity and death in these severely injured patients (Figure 37.1).
HOST IMMUNE FUNCTION
Thermal injury initiates a deleterious pathophysiologic response in every organ system with the extent and duration of organ dysfunction proportionate to the size of the burn. Direct cellular damage is manifested by coagulation necrosis with the depth of tissue destruction determined by the duration of contact and the temperature to which the tissue is exposed. Following a burn, the normal skin barrier to microbial penetration is lost, and the moist, protein-rich avascular eschar of the burn wound provides an excellent culture medium for microorganisms. While destruction of the mechanical barrier of the skin contributes to the increased susceptibility to infection, postburn alterations in immune function also could be of significant importance. Every component of the humoral and cellular limbs of the immune system appears to be affected after thermal injury; the magnitude and duration of dysfunction are proportional to the extent of injury.
During the first weeks after injury, the total white blood cell count is elevated, but peripheral blood lymphocyte counts decrease. Alterations in lymphocyte subpopulations, including reversal of the normal ratio of T helper cells to T suppressor cells, have been described (3,4). Delayed hypersensitivity reactions and peripheral blood lymphocyte proliferation in the mixed lymphocyte reaction are both inhibited following burns. Alterations in interleukin-2 (IL-2) production and IL-2 receptor expression by lymphocytes have been observed with burn injury; a direct correlation has been established between the extent of the burn and the decline of IL-2 production by peripheral lymphocytes (5).
Increased numbers of circulating B lymphocytes are evident early in the postburn course; however, serum immunoglobulin G (IgG) levels decline after burn injury and gradually return to normal over the succeeding 2 to 4 weeks. The association of higher numbers of circulating B cells but reduced serum levels of IgG suggests a defect in the ability of B cells to generate a normal response after burn injury (6). Similar findings have been observed in IgM-producing cells isolated from murine mesenteric lymph nodes and spleens following burns (7). Exogenous administration of IgG to burn patients has been shown to promptly restore normal IgG levels, but exerts no demonstrable effect on the incidence or outcome of infections (8).
Burn injury induces a severity-related shift in the maturity of circulating granulocytes that continues for several weeks after injury. Alterations in granulocyte function, including those of
chemotaxis, adherence, degranulation, oxygen radical production, and complement receptor expression, have been identified (9). Granulocytes isolated from burn patients exhibit increased cytosolic oxidase activity and greater-than-normal oxidase activity after in vitro stimulation, suggesting that these neutrophils are primed and capable of producing more tissue and organ injury (10). Recent investigations have verified elevations of F-actin content and impaired ability to polymerize F-actin in the granulocytes of burn patients when compared with those of controls (11). These alterations can, in part, be responsible for the observed changes in chemotaxis and migration after thermal injury.
chemotaxis, adherence, degranulation, oxygen radical production, and complement receptor expression, have been identified (9). Granulocytes isolated from burn patients exhibit increased cytosolic oxidase activity and greater-than-normal oxidase activity after in vitro stimulation, suggesting that these neutrophils are primed and capable of producing more tissue and organ injury (10). Recent investigations have verified elevations of F-actin content and impaired ability to polymerize F-actin in the granulocytes of burn patients when compared with those of controls (11). These alterations can, in part, be responsible for the observed changes in chemotaxis and migration after thermal injury.
ETIOLOGY OF BURN WOUND INFECTION
Both the nature of the burn wound and microorganism-specific factors influence the rate of microbial proliferation in and penetration of the burn eschar. Burn tissue, rich in coagulated protein and well hydrated by the trans-eschar movement of fluid and serum, creates an excellent microbial culture medium. The eschar is avascular owing to thermal thrombosis of nutrient vessels, limiting both the delivery of systemically administered antibiotics and the migration of phagocytic cells into the burned tissue. Bacterial proliferation in the wound can also be enhanced by such factors as wound maceration, pressure necrosis, and wound desiccation with neo-eschar formation. In addition, secondary impairment of blood flow to the wound could further predispose the patient to invasive infection by curtailing the delivery of oxygen, nutrients, and phagocytic cells to the viable subeschar tissue.
The character of the microbial flora of the burn wound changes with time. The gram-positive organisms that predominate in the early postburn period are replaced by gram-negative organisms by the second week. Without the application of topical antimicrobial agents, the density of bacteria grows progressively, and the microorganisms penetrate the eschar by migration along sweat glands and hair follicles until they reach the eschar/nonviable tissue interface. Additional microbial proliferation occurs in the subeschar space, enhancing the lysis of denatured collagen and sloughing the eschar. If the density and invasiveness of the microorganisms exceed the host’s defense capacity, proliferating organisms in the subeschar space can invade the underlying viable tissue, leading to invasive burn wound infection and even systemic spread to remote tissues and organs. Bacterial invasion is uncommon unless the number of microorganisms exceeds 105/g of biopsy tissue.
Certain strain-specific factors also appear to be important in the pathogenesis of invasive burn wound infection. The production of enzymes, such as collagenase, elastase, protease, and lipase, can enhance the organisms’ ability to penetrate the eschar. Moreover, bacterial motility and antibiotic resistance appear to be important in the development of invasive infection. Effective topical antimicrobial chemotherapy limits intraeschar bacterial proliferation and the attendant risk of invasive infection.
HISTOLOGIC STAGING OF BURN WOUND COLONIZATION AND INFECTION
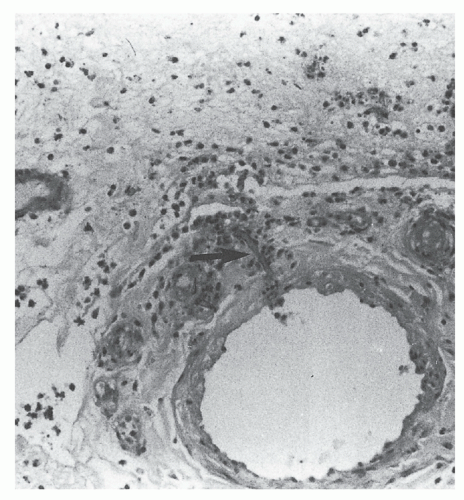
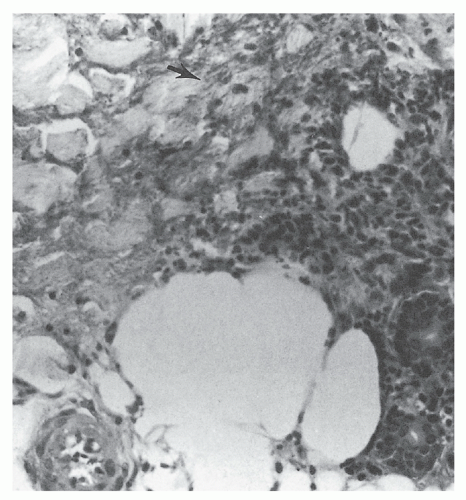
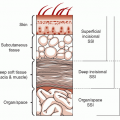
The presence of microorganisms in the nonviable burned tissue, termed colonization, is a distinctly different entity from the presence of microorganisms in viable tissue beneath the burn eschar, termed invasive burn wound infection. Histologic examination of a biopsy of the burn wound and underlying viable tissue is the most rapid and reliable method for differentiating microbial colonization from invasive infection (12). Burn wound biopsy is performed as an intensive care unit or ward procedure. An elliptical biopsy (0.5 × 1.0 cm), which includes the subjacent unburned viable tissue, is obtained by scalpel dissection from an area of burn wound suspected of being infected. Hemostasis is easily achieved by the application of direct pressure or by electrocoagulation. One-half of the specimen is cultured to identify microorganisms and their antimicrobial sensitivities. The remaining half is submitted to the pathology laboratory for histologic examination. Using the rapid section technique, results are available in 3 to 4 hours, whereas a frozen-section technique can yield a diagnosis in 30 minutes, albeit with an attendant 4% falsely negative diagnosis rate (13). The presence of microorganisms in viable tissue confirms the diagnosis of invasive burn wound infection (Figure 37.2). When the organisms are confined to the necrotic eschar or there is suppuration in the subeschar space separating nonviable from viable tissue, the wound is considered to be colonized, not infected (Figure 37.3). Table 37.1 presents a histologic staging scheme for burn wound colonization and infection.
Surface cultures of the burn eschar cannot distinguish colonization from invasive infection. Although commonly used in clinical practice, quantitative bacteriologic cultures of the burn wound correlate poorly with the presence of invasive burn wound infection. When bacteriologic counts are <105/g of biopsy tissue, invasive burn wound infection is rarely present; however, even when quantitative counts exceed 105 organisms/g, histologic examination confirms the presence of invasive infection in <50% of such specimens. Burn wound biopsies can at times yield misleading results, but less commonly than wound cultures. Failing to include viable subeschar tissue in the biopsy specimen or sampling of a noninfected area can limit the usefulness of this technique. Negative biopsy results in the presence of clinical deterioration necessitate reexamination of the burn wound and procurement of biopsy material
from other areas when other sources of systemic infection have been discounted.
from other areas when other sources of systemic infection have been discounted.
TABLE 37.1 Histologic Staging of Burn Wound Colonization and Invasion | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
BURN WOUND MICROBIAL FLORA
Over the past 20 years, significant changes in the microbial ecology of the burn wound have been noted. The recovery of Pseudomonas spp. and other gram-negative bacteria, which were once the most common organisms causing burn wound infection, has markedly declined with improvements in the isolation of patients (14). Consequently, invasive Pseudomonas spp. burn wound infection has essentially disappeared as a complication of burn patients treated in tertiary burn centers (15). Tables 37.2 and 37.3 review all burn wound biopsy results in general and those demonstrating invasive burn wound infection in particular, respectively, during a recent 5-year period at the U.S. Army Institute of Surgical Research and confirm this fact. Patients who have received broad-spectrum antimicrobials for perioperative coverage or treatment of septic complications and whose wounds remain open for many days owing to the extent of the burn are at increased risk of burn wound colonization and infection by yeasts, fungi, and multiple antibiotic-resistant bacteria. The true fungi have replaced bacteria as the most common microbes causing burn wound infection in recent years (16). This predominance of fungal wound infections must be viewed in the context of the marked overall decline in wound infections. Moreover, improperly cared for and neglected burn wounds have the same high risk of bacterial infection as was common in this country several decades ago.
TABLE 37.2 Microorganisms Causing Burn Wound Infection | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree